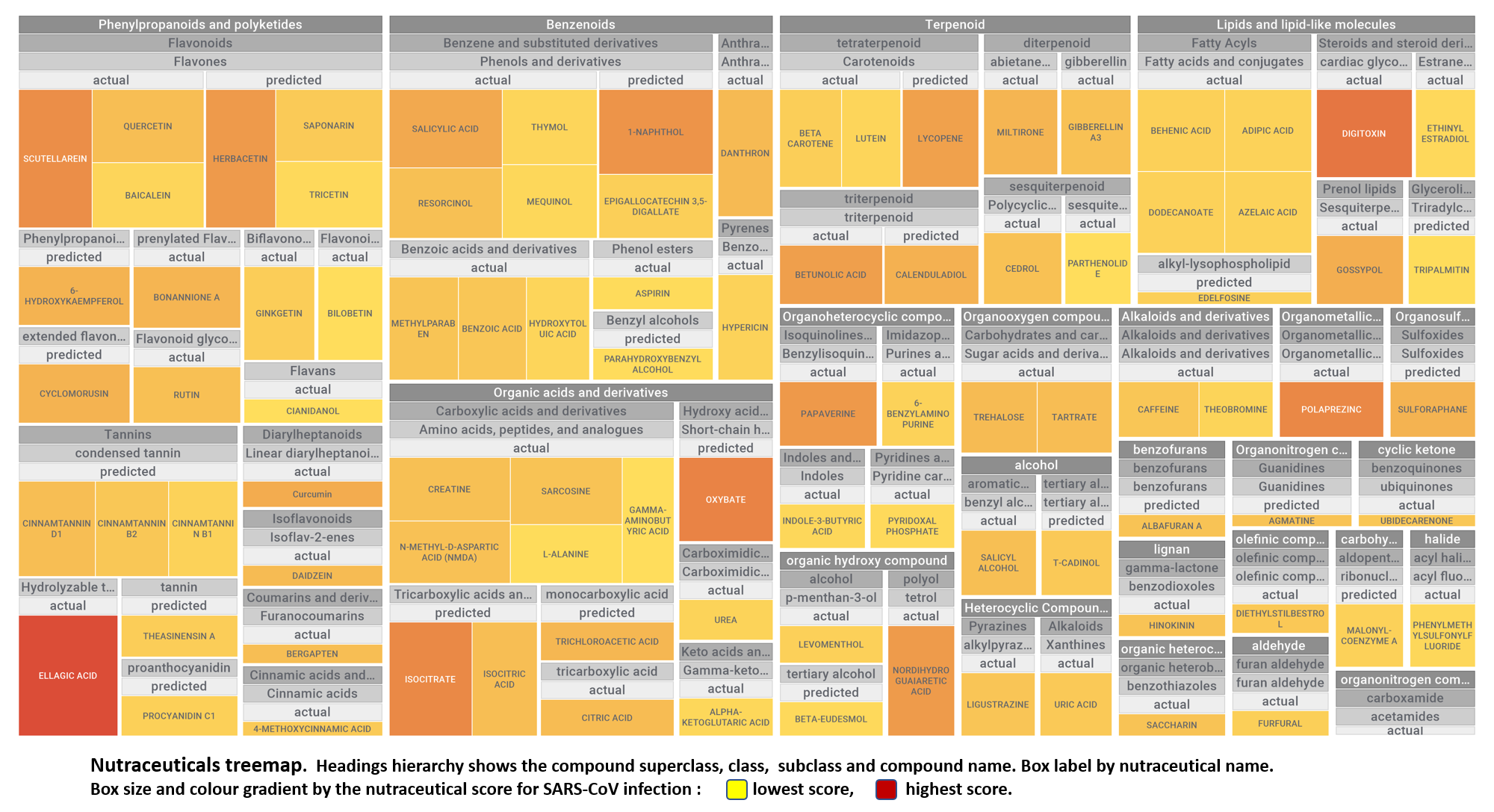
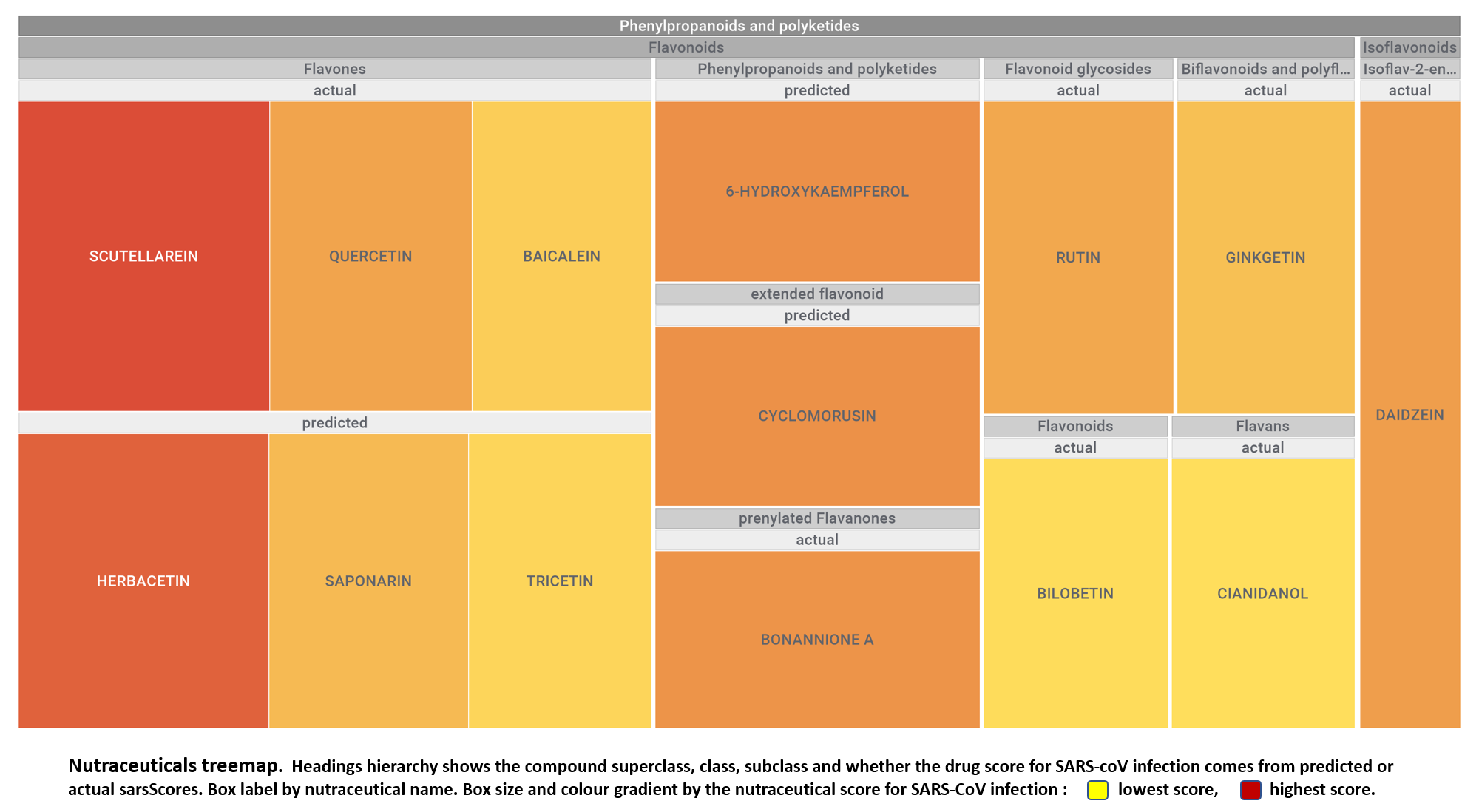
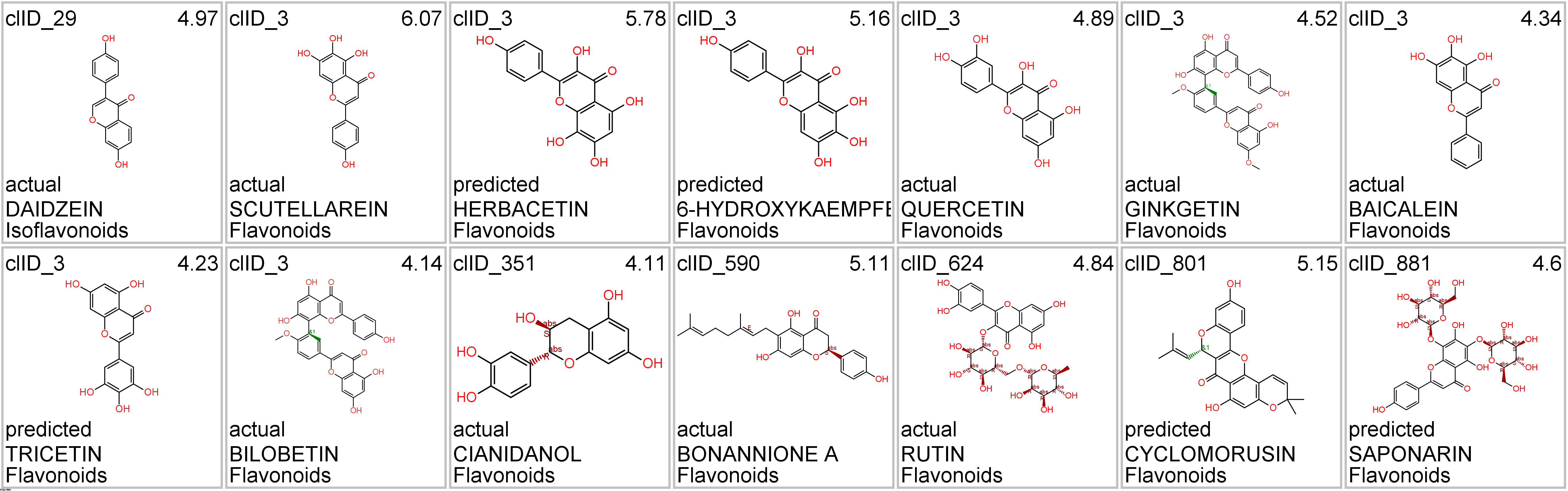
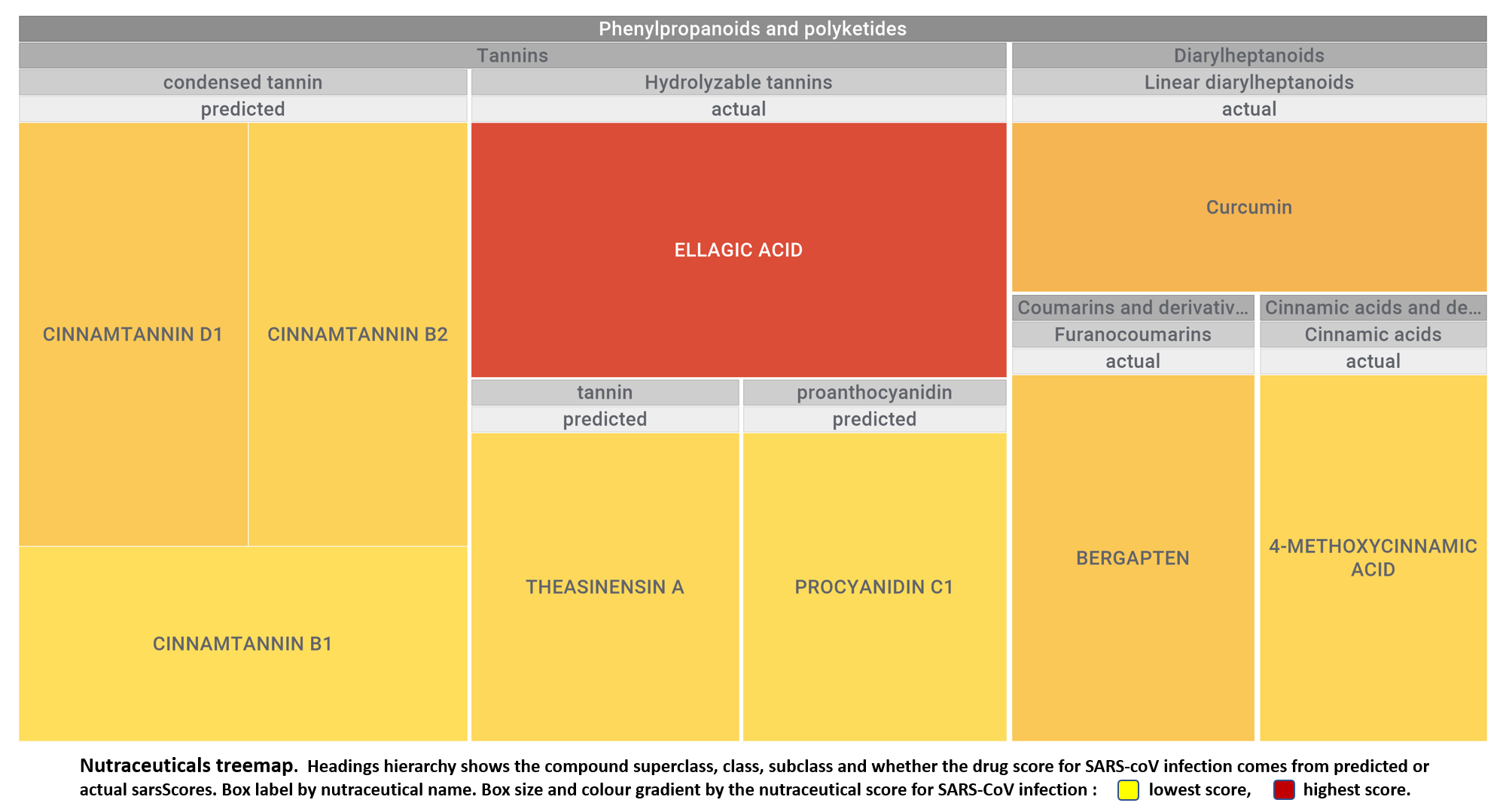
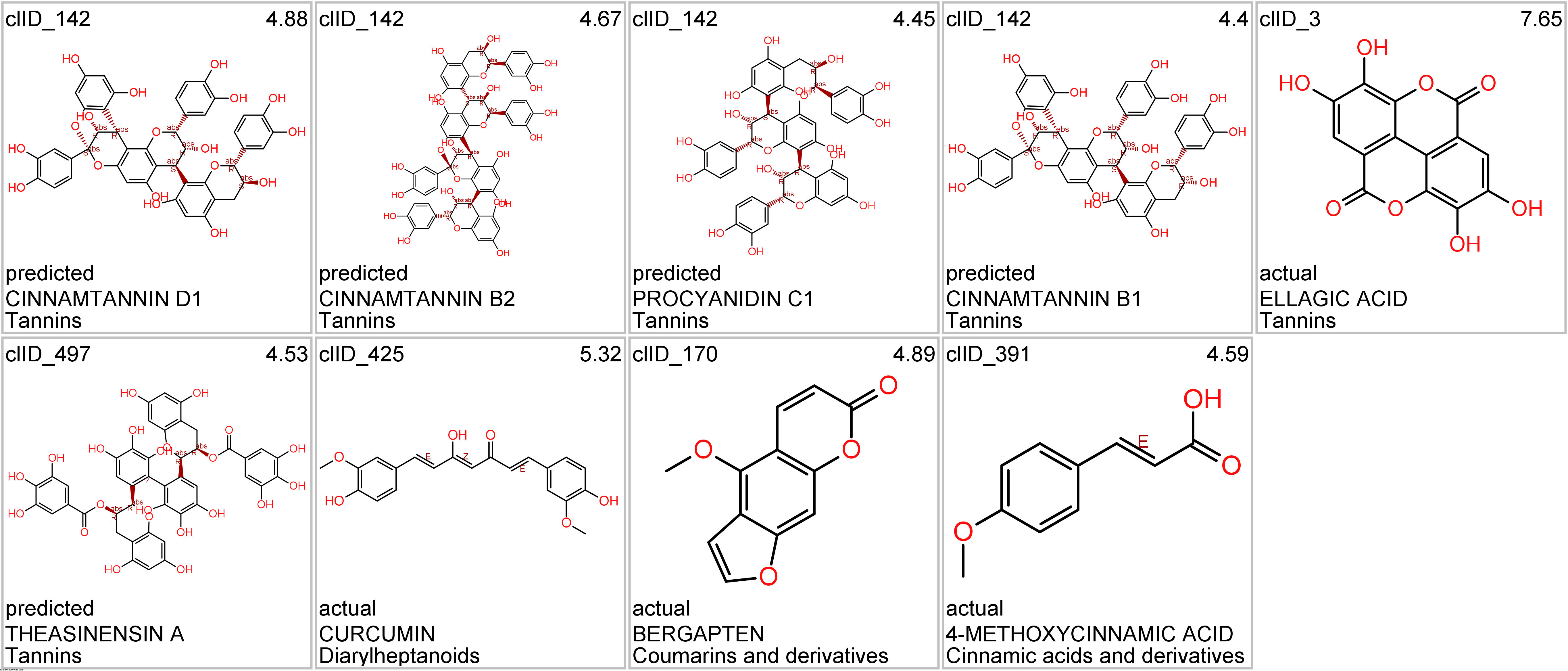
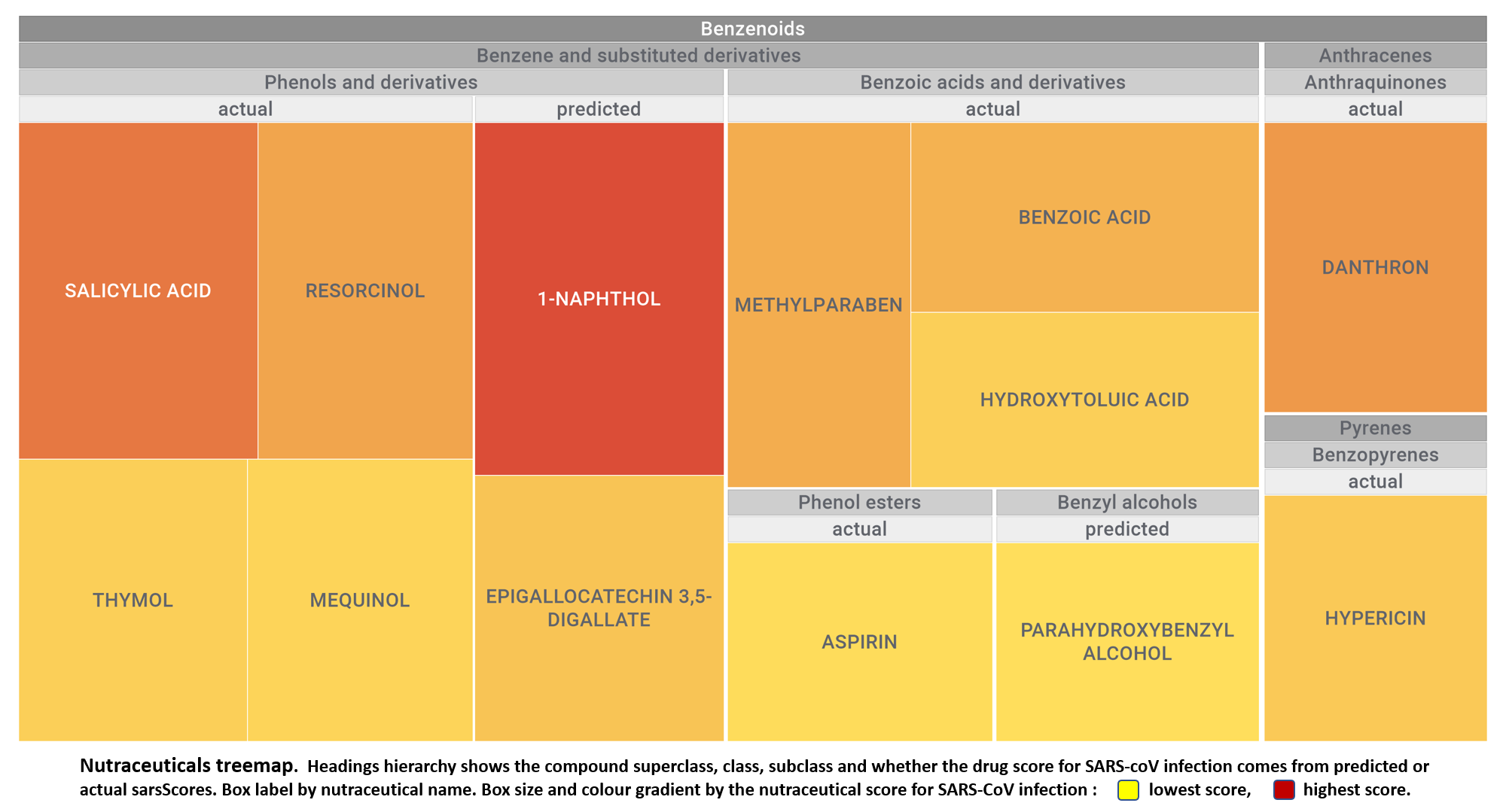
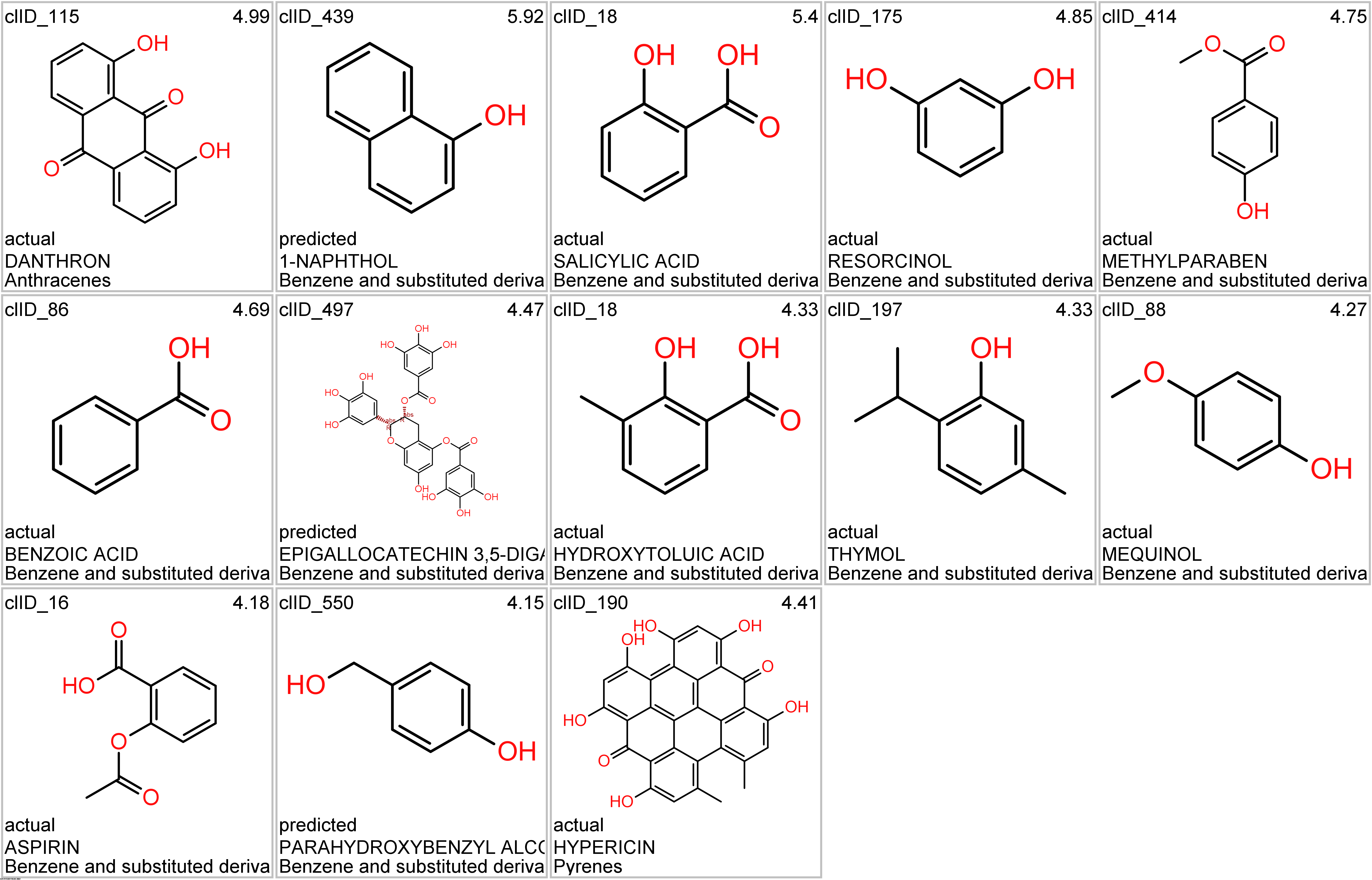
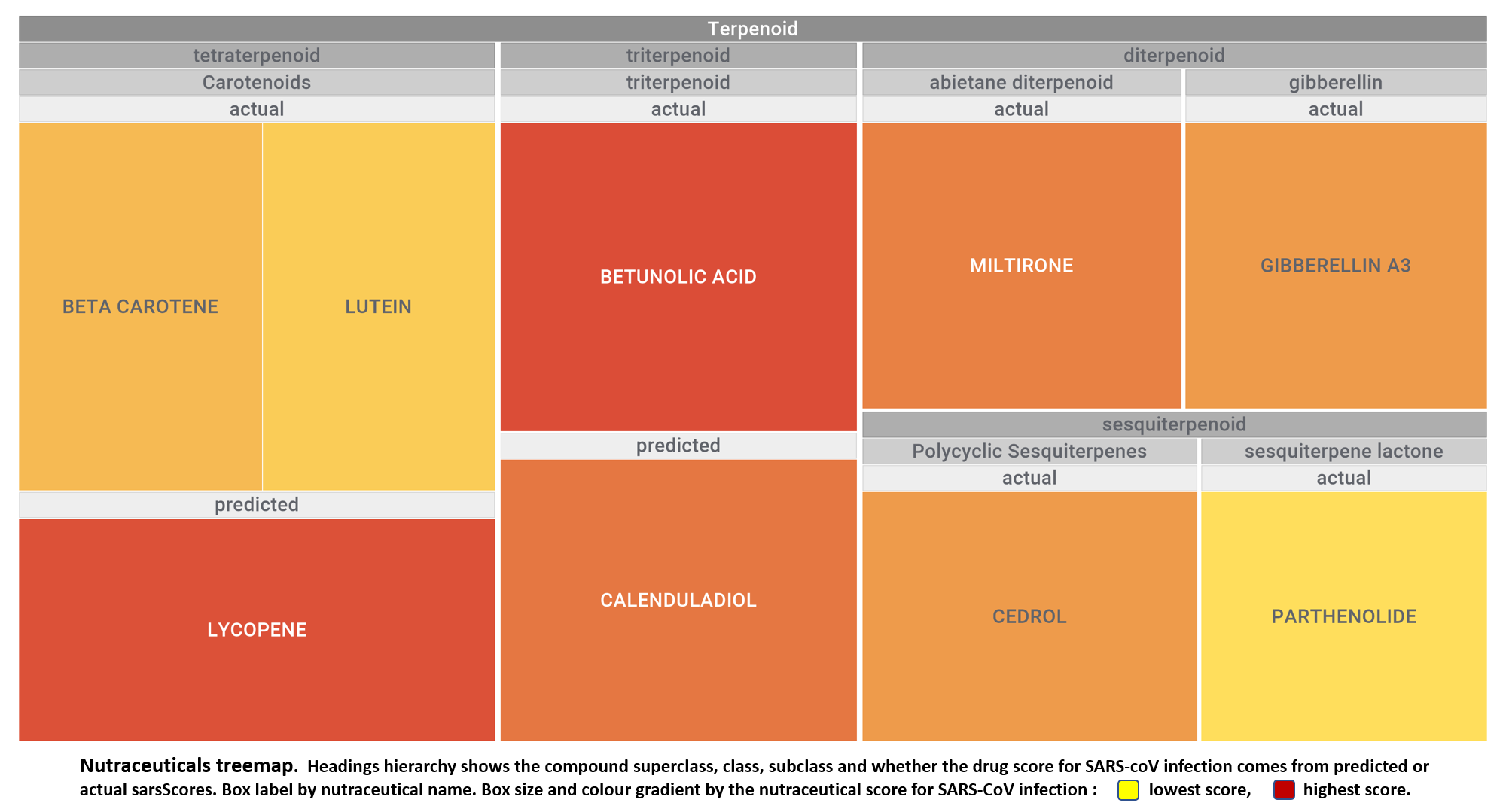
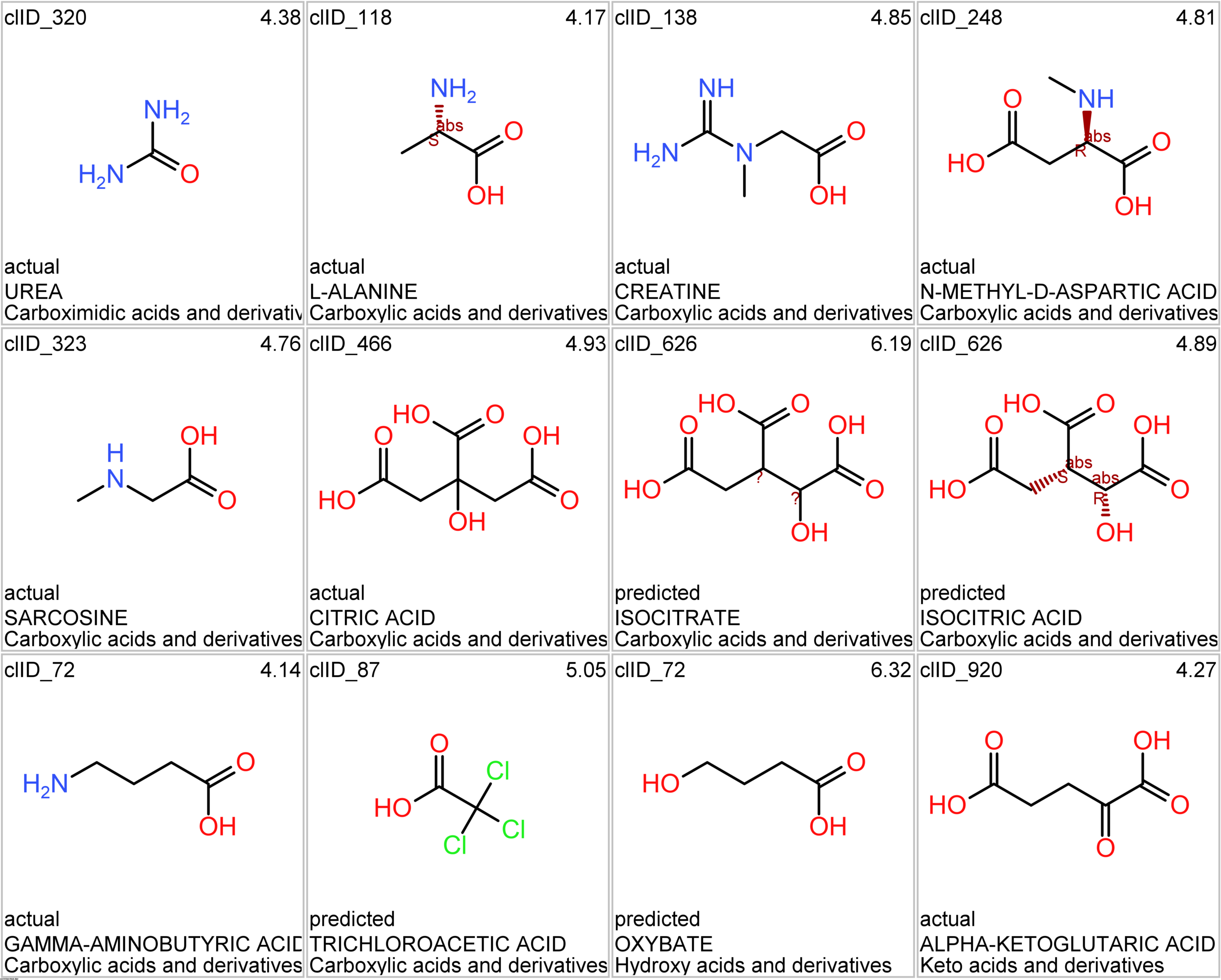
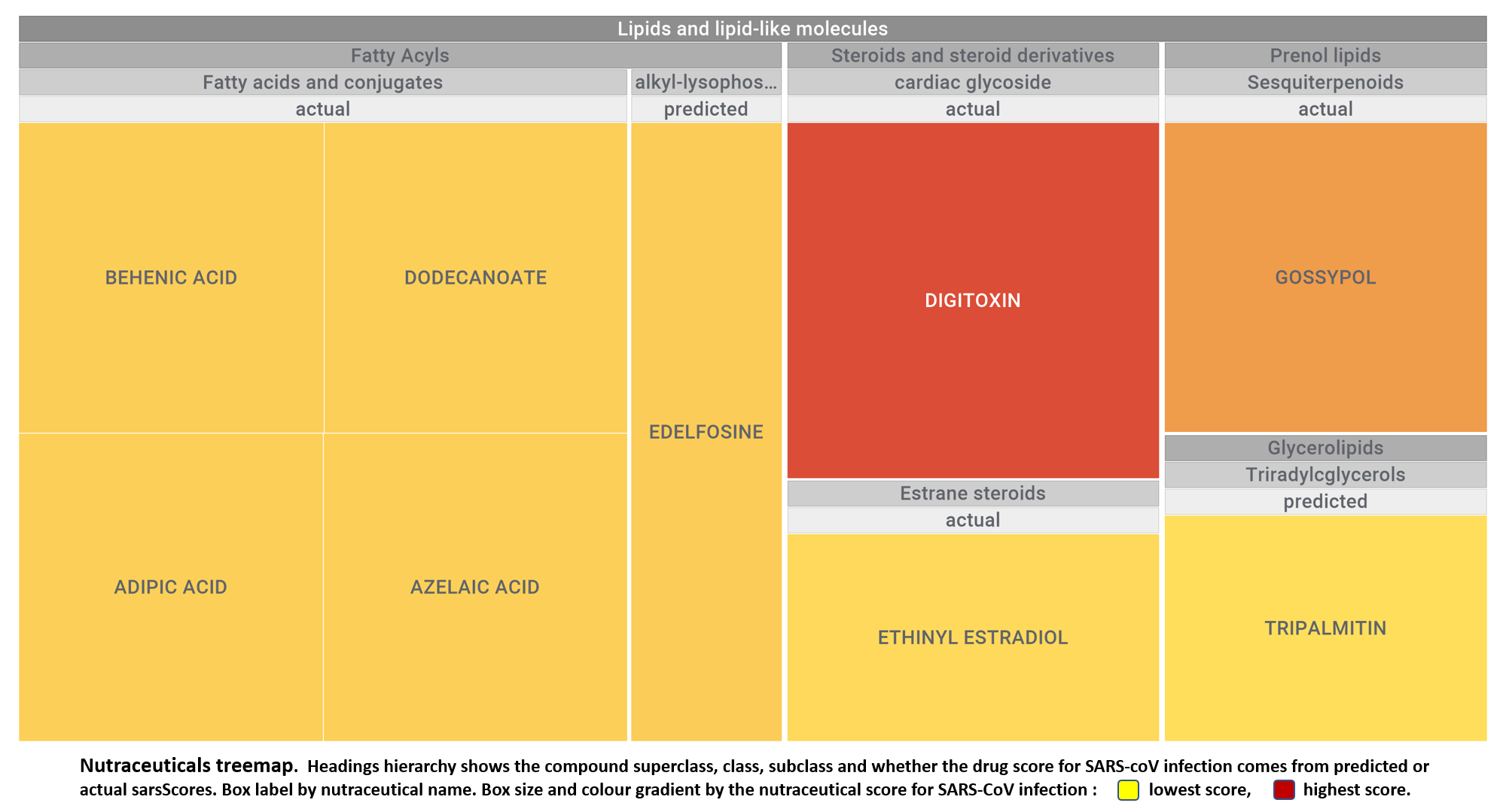
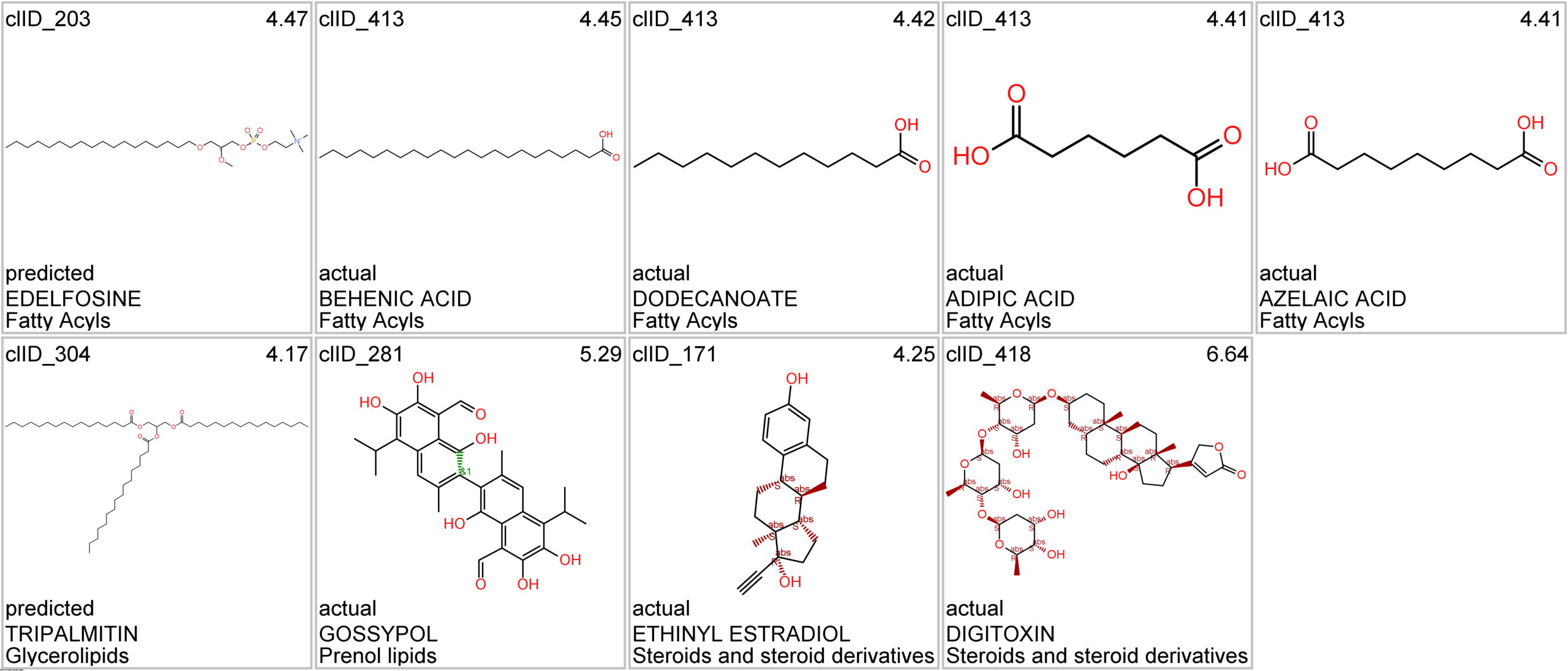
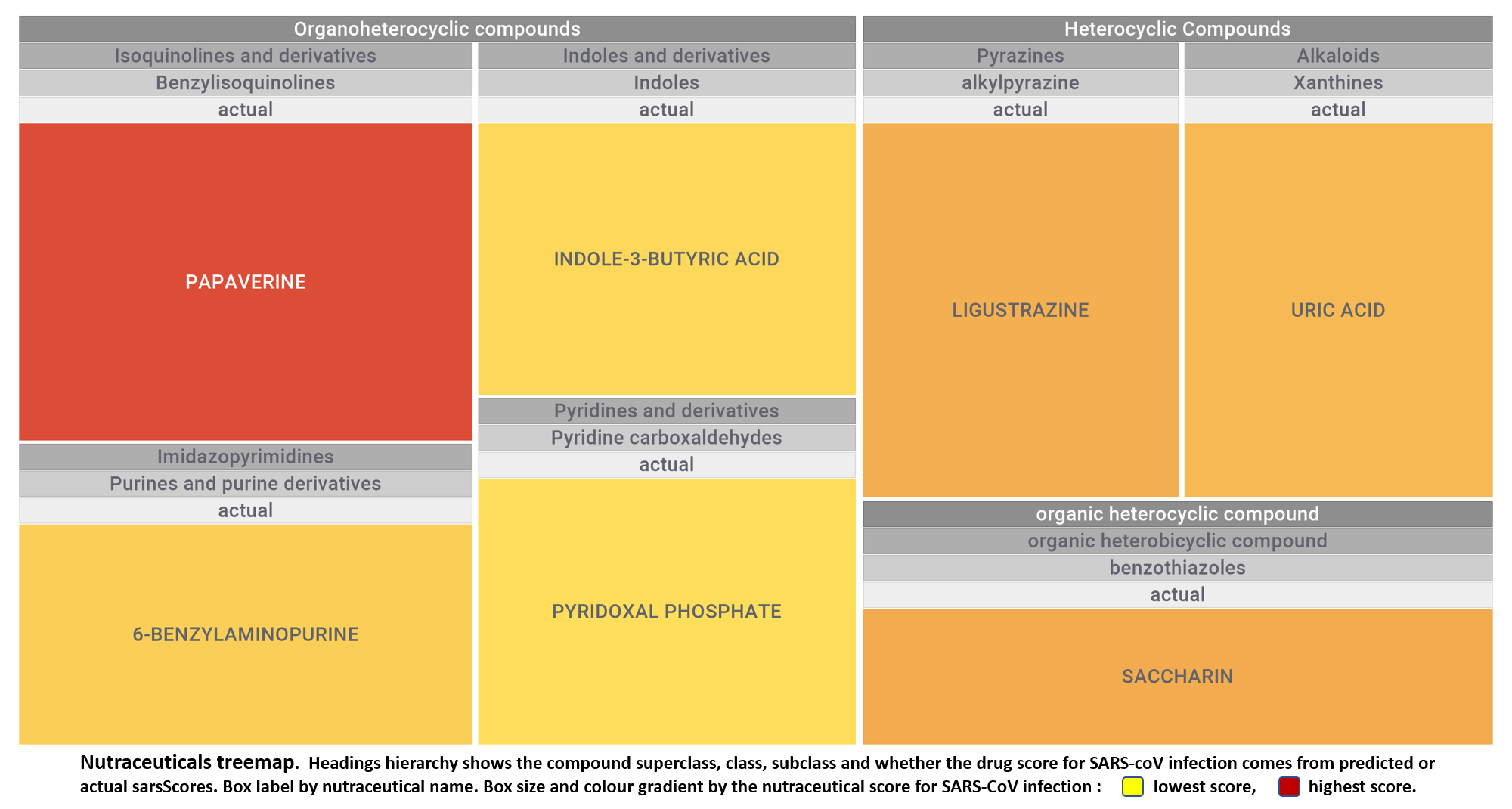
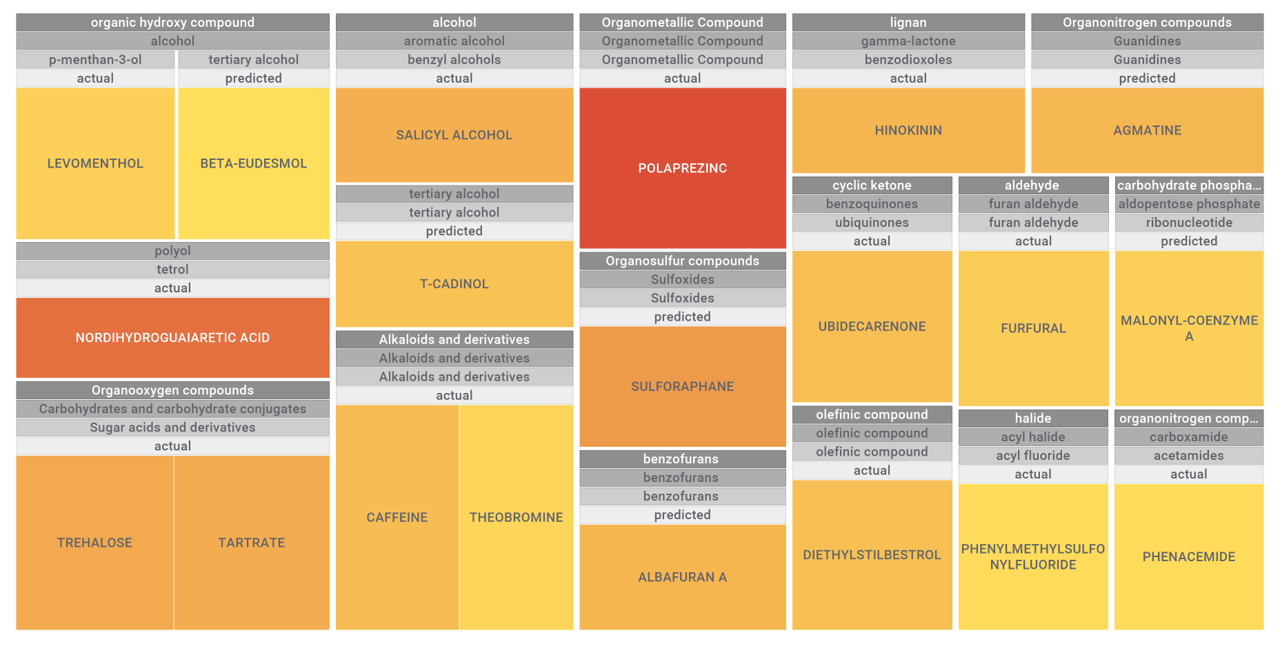
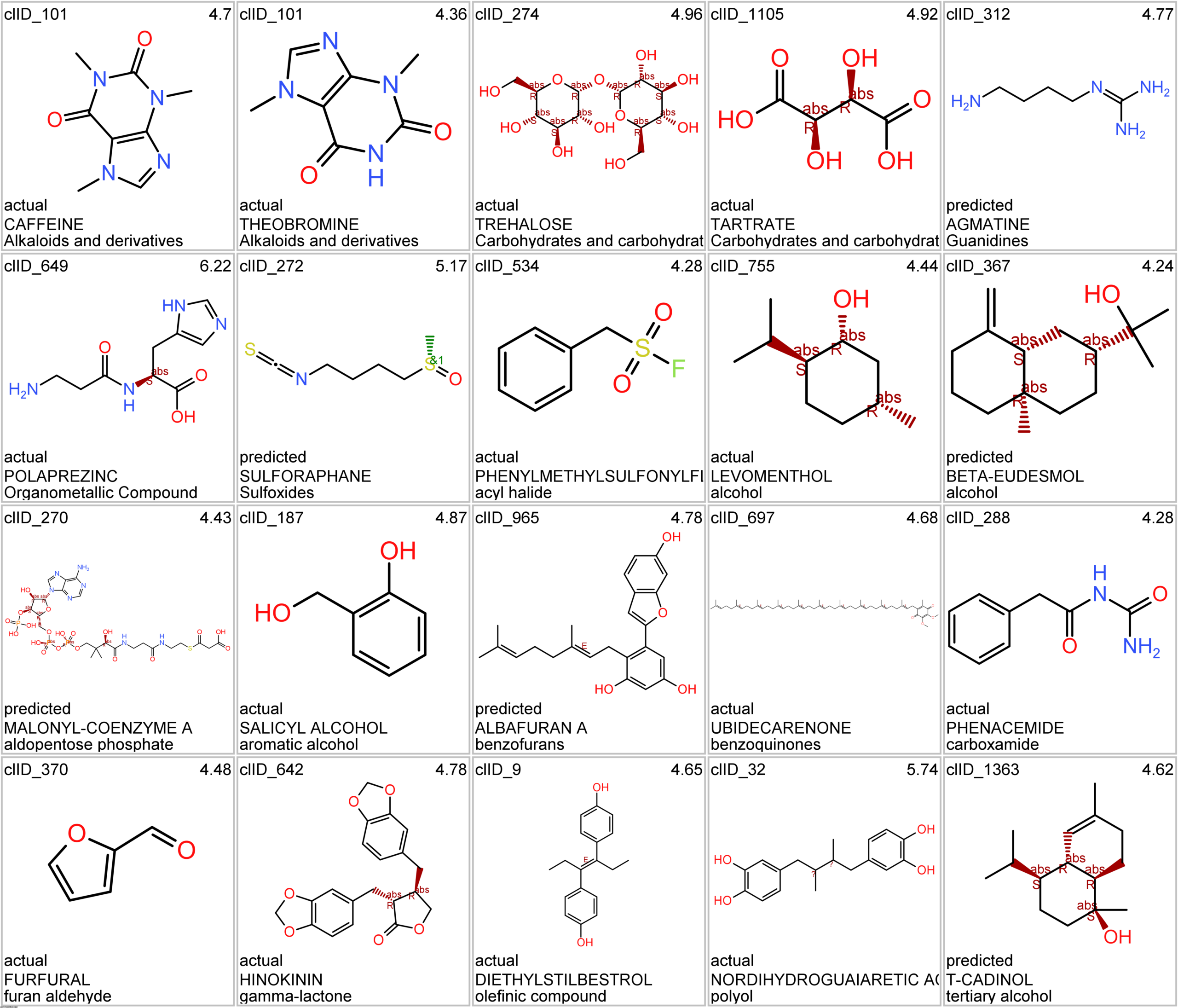
Let’s apply FOODB classification of nutraceuticals to allow a more specific view on each compound family.
Just click on the tabs below to see each class structures and literature support for their role against SARS-CoV infections.
J Enzyme Inhib Med Chem. 2020 Dec;35(1):145-151.
Inhibition of SARS-CoV 3CL protease by flavonoids
Seri Jo 1, Suwon Kim 1, Dong Hae Shin 1, Mi-Sun Kim 1
Free PMC article
Abstract
There were severe panics caused by Severe Acute Respiratory Syndrome (SARS) and Middle-East Respiratory Syndrome-Coronavirus. Therefore, researches targeting these viruses have been required. Coronaviruses (CoVs) have been rising targets of some flavonoids. The antiviral activity of some flavonoids against CoVs is presumed directly caused by inhibiting 3C-like protease (3CLpro). Here, we applied a flavonoid library to systematically probe inhibitory compounds against SARS-CoV 3CLpro. Herbacetin, rhoifolin and pectolinarin were found to efficiently block the enzymatic activity of SARS-CoV 3CLpro. The interaction of the three flavonoids was confirmed using a tryptophan-based fluorescence method, too. An induced-fit docking analysis indicated that S1, S2 and S3′ sites are involved in binding with flavonoids. The comparison with previous studies showed that Triton X-100 played a critical role in objecting false positive or overestimated inhibitory activity of flavonoids. With the systematic analysis, the three flavonoids are suggested to be templates to design functionally improved inhibitors.
Chem Biol Interact. 2020 Sep 1; 328: 109211.
Published online 2020 Jul 28. doi: 10.1016/j.cbi.2020.109211
Roles of flavonoids against coronavirus infection
Maria Russo,1 Stefania Moccia,1 Carmela Spagnuolo, Idolo Tedesco, and Gian Luigi Russo∗
Author information Article notes Copyright and License information Disclaimer
Abstract
In terms of public health, the 21st century has been characterized by coronavirus pandemics: in 2002-03 the virus SARS-CoV caused SARS; in 2012 MERS-CoV emerged and in 2019 a new human betacoronavirus strain, called SARS-CoV-2, caused the unprecedented COVID-19 outbreak. During the course of the current epidemic, medical challenges to save lives and scientific research aimed to reveal the genetic evolution and the biochemistry of the vital cycle of the new pathogen could lead to new preventive and therapeutic strategies against SARS-CoV-2. Up to now, there is no cure for COVID-19 and waiting for an efficacious vaccine, the development of “savage” protocols, based on “old” anti-inflammatory and anti-viral drugs represents a valid and alternative therapeutic approach. As an alternative or additional therapeutic/preventive option, different in silico and in vitro studies demonstrated that small natural molecules, belonging to polyphenol family, can interfere with various stages of coronavirus entry and replication cycle. Here, we reviewed the capacity of well-known (e.g. quercetin, baicalin, luteolin, hesperetin, gallocatechin gallate, epigallocatechin gallate) and uncommon (e.g. scutellarein, amentoflavone, papyriflavonol A) flavonoids, secondary metabolites widely present in plant tissues with antioxidant and anti-microbial functions, to inhibit key proteins involved in coronavirus infective cycle, such as PLpro, 3CLpro, NTPase/helicase. Due to their pleiotropic activities and lack of systemic toxicity, flavonoids and their derivative may represent target compounds to be tested in future clinical trials to enrich the drug arsenal against coronavirus infections.In terms of public health, the 21st century has been characterized by coronavirus pandemics: in 2002-03 the virus SARS-CoV caused SARS; in 2012 MERS-CoV emerged and in 2019 a new human betacoronavirus strain, called SARS-CoV-2, caused the unprecedented COVID-19 outbreak. During the course of the current epidemic, medical challenges to save lives and scientific research aimed to reveal the genetic evolution and the biochemistry of the vital cycle of the new pathogen could lead to new preventive and therapeutic strategies against SARS-CoV-2. Up to now, there is no cure for COVID-19 and waiting for an efficacious vaccine, the development of “savage” protocols, based on “old” anti-inflammatory and anti-viral drugs represents a valid and alternative therapeutic approach. As an alternative or additional therapeutic/preventive option, different in silico and in vitro studies demonstrated that small natural molecules, belonging to polyphenol family, can interfere with various stages of coronavirus entry and replication cycle. Here, we reviewed the capacity of well-known (e.g. quercetin, baicalin, luteolin, hesperetin, gallocatechin gallate, epigallocatechin gallate) and uncommon (e.g. scutellarein, amentoflavone, papyriflavonol A) flavonoids, secondary metabolites widely present in plant tissues with antioxidant and anti-microbial functions, to inhibit key proteins involved in coronavirus infective cycle, such as PLpro, 3CLpro, NTPase/helicase. Due to their pleiotropic activities and lack of systemic toxicity, flavonoids and their derivative may represent target compounds to be tested in future clinical trials to enrich the drug arsenal against coronavirus infections.
Bioorg Med Chem Lett. 2012 Jun 15;22(12):4049-54.
Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13
Mi-Sun Yu 1, June Lee, Jin Moo Lee, Younggyu Kim, Young-Won Chin, Jun-Goo Jee, Young-Sam Keum, Yong-Joo Jeong
Free PMC article
Abstract
Severe acute respiratory syndrome (SARS) is an infectious disease with a strong potential for transmission upon close personal contact and is caused by the SARS-coronavirus (CoV). However, there are no natural or synthetic compounds currently available that can inhibit SARS-CoV. We examined the inhibitory effects of 64 purified natural compounds against the activity of SARS helicase, nsP13, and the hepatitis C virus (HCV) helicase, NS3h, by conducting fluorescence resonance energy transfer (FRET)-based double-strand (ds) DNA unwinding assay or by using a colorimetry-based ATP hydrolysis assay. While none of the compounds, examined in our study inhibited the DNA unwinding activity or ATPase activity of human HCV helicase protein, we found that myricetin and scutellarein potently inhibit the SARS-CoV helicase protein in vitro by affecting the ATPase activity, but not the unwinding activity, nsP13. In addition, we observed that myricetin and scutellarein did not exhibit cytotoxicity against normal breast epithelial MCF10A cells. Our study demonstrates for the first time that selected naturally-occurring flavonoids, including myricetin and scultellarein might serve as SARS-CoV chemical inhibitors.
J Enzyme Inhib Med Chem. 2021; 36(1): 497–503.
Published online 2021 Jan 24. doi: 10.1080/14756366.2021.1873977
Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro
Hongbo Liu,a Fei Ye,b Qi Sun,a Hao Liang,a Chunmei Li,c Siyang Li,c Roujian Lu,b Baoying Huang,b Wenjie Tan,b and Luhua Laia,c
Abstract
COVID-19 has become a global pandemic and there is an urgent call for developing drugs against the virus (SARS-CoV-2). The 3C-like protease (3CLpro) of SARS-CoV-2 is a preferred target for broad spectrum anti-coronavirus drug discovery. We studied the anti-SARS-CoV-2 activity of S. baicalensis and its ingredients. We found that the ethanol extract of S. baicalensis and its major component, baicalein, inhibit SARS-CoV-2 3CLpro activity in vitro with IC50’s of 8.52 µg/ml and 0.39 µM, respectively. Both of them inhibit the replication of SARS-CoV-2 in Vero cells with EC50’s of 0.74 µg/ml and 2.9 µM, respectively. While baicalein is mainly active at the viral post-entry stage, the ethanol extract also inhibits viral entry. We further identified four baicalein analogues from other herbs that inhibit SARS-CoV-2 3CLpro activity at µM concentration. All the active compounds and the S. baicalensis extract also inhibit the SARS-CoV 3CLpro, demonstrating their potential as broad-spectrum anti-coronavirus drugs.COVID-19 has become a global pandemic and there is an urgent call for developing drugs against the virus (SARS-CoV-2). The 3C-like protease (3CLpro) of SARS-CoV-2 is a preferred target for broad spectrum anti-coronavirus drug discovery. We studied the anti-SARS-CoV-2 activity of S. baicalensis and its ingredients. We found that the ethanol extract of S. baicalensis and its major component, baicalein, inhibit SARS-CoV-2 3CLpro activity in vitro with IC50’s of 8.52 µg/ml and 0.39 µM, respectively. Both of them inhibit the replication of SARS-CoV-2 in Vero cells with EC50’s of 0.74 µg/ml and 2.9 µM, respectively. While baicalein is mainly active at the viral post-entry stage, the ethanol extract also inhibits viral entry. We further identified four baicalein analogues from other herbs that inhibit SARS-CoV-2 3CLpro activity at µM concentration. All the active compounds and the S. baicalensis extract also inhibit the SARS-CoV 3CLpro, demonstrating their potential as broad-spectrum anti-coronavirus drugs.
Quercetin: Antiviral Significance and Possible COVID-19 Integrative Considerations
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
First Published December 3, 2020 Review Article
https://doi.org/10.1177/1934578X20976293
Abstract
Quercetin, a naturally occurring dietary flavonoid, is well known to ameliorate chronic diseases and aging processes in humans, and its antiviral properties have been investigated in numerous studies. In silico and in vitro studies demonstrated that quercetin can interfere with various stages of the coronavirus entry and replication cycle such as PLpro, 3CLpro, and NTPase/helicase. Due to its pleiotropic activities and lack of systemic toxicity, quercetin and its derivatives may represent target compounds to be tested in future clinical trials to enrich the drug arsenal against coronavirus infections. There is evidence that quercetin in combination with, for example, vitamins C and D, may exert a synergistic antiviral action that may provide either an alternative or additional therapeutic/preventive option due to overlapping antiviral and immunomodulatory properties. This review summarizes the antiviral significance of quercetin and proposes a possible strategy for the effective utilization of natural polyphenols in our daily diet for the prevention of viral infection.
J Food Biochem. 2020 Aug 11 : e13432.
doi: 10.1111/jfbc.13432 [Epub ahead of print]
Tannins inhibit SARS‐CoV‐2 through binding with catalytic dyad residues of 3CLpro: An in silico approach with 19 structural different hydrolysable tannins
Ibrahim Khalifa, 1 Wei Zhu, 2 Hammad Hamed Hammad Mohammed, 2 , 3 Kunal Dutta, 4 and Chunmei Li
Abstract
Coronavirus epidemic 2019 (COVID‐19), instigated by SARS‐CoV‐2 virus, is recently raising worldwide and inspiring global health worries. The main 3‐chymotrypsin‐like cysteine protease (3CLPro) enzyme of SARS‐CoV‐2, which operates its replication, could be used as a medication discovery point. We therefore theoretically studied and docked the effects of 19 hydrolysable tannins on SARS‐CoV‐2 by assembling with the catalytic dyad residues of its 3CLpro using molecular operating environment (MOE 09). Results discovered that pedunculagin, tercatain, and castalin intensely interacted with the receptor binding site and catalytic dyad (Cys145 and His41) of SARS‐CoV‐2. Our analyses estimated that the top three hits might serve as potential inhibitor of SARS‐CoV‐2 leading molecules for additional optimization and drug development process to combat COVID‐19. This study unleashed that tannins with specific structure could be utilized as natural inhibitors against COVID‐19.
Practical applications
The 3CLPro controls SARS‐CoV‐2 copying and manages its life series, which was targeted in case of SARS‐CoV and MERS‐CoV coronavirus. About 19 hydrolysable tannins were computed against 3CLpro of SARS‐CoV‐2. Pedunculagin, tercatain, and castalin interacted with Cys145 and His41 of SARS‐CoV‐2‐3CLpro. Pedunculagin‐SARS‐CoV‐2‐3CLpro remain stable, with no obvious fluctuations. We predicted that the understandings gained in the current research may evidence valued for discovering and unindustrialized innovative natural inhibitors for COVID‐19 in the nearby future.
Abstract
About 19 hydrolyzable tannins were computed against 3CLpro enzyme of 2019‐nCoV. It was found that pedunculagin, tercatain, and castalin interacted with Cys145 and His41 of 2019‐nCoV‐3CLpro. Likewise, pedunculagin‐2019‐nCoV‐3CLpro remain stable, with no obvious fluctuations. We predicted that the understandings obtained in the current study may evidence valued for discovering and unindustrialized novel natural anti‐COVID‐19 therapeutic agents in the near future.
In silico investigation of saponins and tannins as potential inhibitors of SARS-CoV-2 main protease (Mpro)
- Victoria Adeola Falade, Temitope Isaac Adelusi, Ibrahim Olaide Adedotun, Misbaudeen Abdul-Hammed, Teslim Alabi Lawal & Saheed Alabi Agboluaje
In Silico Pharmacology volume 9, Article number: 9 (2021) Cite this article
https://link.springer.com/article/10.1007/s40203-020-00071-w
Abstract
It is no longer news that a novel strain of coronavirus named SARS-CoV-2 is ravaging the health sector worldwide, several attempts have been made to curtail this pandemic via repurposing of old drugs but at the present, available drugs are not adequately effective. Over the years, plant phytochemicals are increasingly becoming alternative sources of antimicrobial agents with novel mechanisms of action and limited side effects compared to synthetic drugs. Isolated saponins and tannins were evaluated for antiviral activity against SARS-CoV-2 (Mpro) via Molecular Docking and it was observed that a handsome number of the phytochemicals had binding affinities much better than Remdesivir, Dexamethasone, and N3 inhibitor which were used as the standards in this study. Further investigation of drug-likeness, ADMET profile, PASS profile, oral bioavailability, bioactivity, binding mode, and molecular interactions of these phytochemicals revealed that binding affinity alone is not enough to justify the potency of a molecule in the drug discovery process, as only 4 among the screened compounds passed all the analyses and are identified as potential inhibitors of SARS-CoV-2 (Mpro). This preliminary study thereby recommends Ellagic acid (− 8.4 kcal/mol), Arjunic Acid (− 8.1 kcal/mol), Theasapogenol B (− 8.1 kcal/mol), and Euscaphic Acid (− 8.0 kcal/mol) as potential inhibitors of SARS-CoV-2 (Mpro) with better pharmacokinetics and bioavailability compared to Remdesivir which is currently used compassionately.
Am J Cancer Res. 2020; 10(12): 4538–4546. PMID: 33415017
Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease
Shao-Chun Wang,1,2,3,4,5,6,* Yeh Chen,3,4,7,* Yu-Chuan Wang,3,4,7 Wei-Jan Wang,3,4,8 Chia-Shin Yang,3,4,7 Chia-Ling Tsai,3,4,7 Mei-Hui Hou,3,4,7 Hsiao-Fan Chen,3,4 Yi-Chun Shen,3,4 and Mien-Chie Hung1,2,3,4,5
Abstract
The cell surface protein TMPRSS2 (transmembrane protease serine 2) is an androgen-responsive serine protease important for prostate cancer progression and therefore an attractive therapeutic target. Besides its role in tumor biology, TMPRSS2 is also a key player in cellular entry by the SARS-CoV viruses. The COVID-19 pandemic caused by the coronavirus SARS-CoV-2 has resulted in huge losses in socio-economy, culture, and human lives for which safe and effective cures are highly demanded. The main protease (Mpro/3CLpro) of SARS-CoV-2 is a critical enzyme for viral propagation in host cells and, like TMPRSS2, has been exploited for treatment of the infectious disease. Numerous natural compounds abundant in common fruits have been suggested with anti-coronavirus infection in the previous outbreaks of SARS-CoV. Here we show that screening of these compounds identified tannic acid a potent inhibitor of both SARS-CoV-2 Mpro and TMPRSS2. Molecular analysis demonstrated that tannic acid formed a thermodynamically stable complex with the two proteins at a KD of 1.1 μM for Mpro and 1.77 μM for TMPRSS2. Tannic acid inhibited the activities of the two proteases with an IC50 of 13.4 μM for Mpro and 2.31 μM for TMPRSS2. Mpro protein. Consistently, functional assays using the virus particles pseudotyped (Vpp) of SARS-CoV2-S demonstrated that tannic acid suppressed viral entry into cells. Thus, our results demonstrate that tannic acid has high potential of developing anti-COVID-19 therapeutics as a potent dual inhibitor of two independent enzymes essential for SARS-CoV-2 infection.
The cell surface protein TMPRSS2 (transmembrane protease serine 2) is an androgen-responsive serine protease important for prostate cancer progression and therefore an attractive therapeutic target. Besides its role in tumor biology, TMPRSS2 is also a key player in cellular entry by the SARS-CoV viruses. The COVID-19 pandemic caused by the coronavirus SARS-CoV-2 has resulted in huge losses in socio-economy, culture, and human lives for which safe and effective cures are highly demanded. The main protease (Mpro/3CLpro) of SARS-CoV-2 is a critical enzyme for viral propagation in host cells and, like TMPRSS2, has been exploited for treatment of the infectious disease. Numerous natural compounds abundant in common fruits have been suggested with anti-coronavirus infection in the previous outbreaks of SARS-CoV. Here we show that screening of these compounds identified tannic acid a potent inhibitor of both SARS-CoV-2 Mpro and TMPRSS2. Molecular analysis demonstrated that tannic acid formed a thermodynamically stable complex with the two proteins at a KD of 1.1 μM for Mpro and 1.77 μM for TMPRSS2. Tannic acid inhibited the activities of the two proteases with an IC50 of 13.4 μM for Mpro and 2.31 μM for TMPRSS2. Mpro protein. Consistently, functional assays using the virus particles pseudotyped (Vpp) of SARS-CoV2-S demonstrated that tannic acid suppressed viral entry into cells. Thus, our results demonstrate that tannic acid has high potential of developing anti-COVID-19 therapeutics as a potent dual inhibitor of two independent enzymes essential for SARS-CoV-2 infection.
Antiviral Res. 2009 Apr; 82(1): 73–81.
Published online 2009 Feb 11. doi: 10.1016/j.antiviral.2009.02.001
Procyanidins and butanol extract of Cinnamomi Cortex inhibit SARS-CoV infection
Min Zhuang,a,b Hong Jiang,c Yasuhiro Suzuki,a Xiaoguang Li,a Peng Xiao,a Takashi Tanaka,d Hong Ling,b Baofeng Yang,e Hiroki Saitoh,a Lianfeng Zhang,c Chuan Qin,c Kazuo Sugamura,f and Toshio Hattoria,⁎
Abstract
We found that the butanol fraction of Cinnamomi Cortex (CC/Fr.2) showed moderate inhibitory activity in wild-type severe acute respiratory syndrome coronavirus (wtSARS-CoV) and HIV/SARS-CoV S pseudovirus infections. The inhibition on pseudovirus was also seen in cells pretreated with the CC and CC/Fr.2 (IC50S, 283.4 ± 16.3 and 149.5 ± 13.5 μg/ml, respectively), however the highest activities on wtSARS-CoV were observed when the viruses were treated by the extracts before challenging (IC50S, 43.1 ± 2.8 and 7.8 ± 0.3 μg/ml; SIs, 8.4 and 23.1, respectively). Among the compounds fractionated from CC, procyanidin A2 and procyanidin B1 showed moderate anti-wtSARS-CoV activity (IC50S, 29.9 ± 3.3 and 41.3 ± 3.4 μM; SIs, 37.35 and 15.69, respectively). We also sought to determine whether they could interfere with the clathrin-dependent endocytosis pathway using transferrin receptor (TfR) as an indicator. CC/Fr.2 inhibited the internalization of TfR but the procyanidins did not. Taken together, CC/Fr.2 contains unknown substances, that could inhibit the infection, probably by interfering with endocytosis, and it also contains procyanidins that did not inhibit the internalization but inhibited the infection. Therefore, CC extracts contain anti-virus activities that act through distinct mechanisms according to differences in the compounds or mixtures.We found that the butanol fraction of Cinnamomi Cortex (CC/Fr.2) showed moderate inhibitory activity in wild-type severe acute respiratory syndrome coronavirus (wtSARS-CoV) and HIV/SARS-CoV S pseudovirus infections. The inhibition on pseudovirus was also seen in cells pretreated with the CC and CC/Fr.2 (IC50S, 283.4 ± 16.3 and 149.5 ± 13.5 μg/ml, respectively), however the highest activities on wtSARS-CoV were observed when the viruses were treated by the extracts before challenging (IC50S, 43.1 ± 2.8 and 7.8 ± 0.3 μg/ml; SIs, 8.4 and 23.1, respectively). Among the compounds fractionated from CC, procyanidin A2 and procyanidin B1 showed moderate anti-wtSARS-CoV activity (IC50S, 29.9 ± 3.3 and 41.3 ± 3.4 μM; SIs, 37.35 and 15.69, respectively). We also sought to determine whether they could interfere with the clathrin-dependent endocytosis pathway using transferrin receptor (TfR) as an indicator. CC/Fr.2 inhibited the internalization of TfR but the procyanidins did not. Taken together, CC/Fr.2 contains unknown substances, that could inhibit the infection, probably by interfering with endocytosis, and it also contains procyanidins that did not inhibit the internalization but inhibited the infection. Therefore, CC extracts contain anti-virus activities that act through distinct mechanisms according to differences in the compounds or mixtures.
A phytochemical-based medication search for the SARS-CoV-2 infection by molecular docking models towards spike glycoproteins and main proteases†
Anju Choorakottayil Pushkaran,a Prajeesh Nath EN,b Anu R. Melge,a Rammanohar Puthiyedath b and C. Gopi Mohan *a
https://pubs.rsc.org/en/content/articlelanding/2021/ra/d0ra10458b#!divAbstract
Abstract
Identifying best bioactive phytochemicals from different medicinal plants using molecular docking techniques demonstrates a potential pre-clinical compound discovery against SARS-CoV-2 viral infection. The in silico screening of bioactive phytochemicals with the two druggable targets of SARS-CoV-2 by simple precision/extra precision molecular docking methods was used to compute binding affinity at its active sites. phyllaemblicin and cinnamtannin class of phytocompounds showed a better binding affinity range (−9.0 to −8.0 kcal mol−1) towards both these SARS-CoV-2 targets; the corresponding active site residues in the spike protein were predicted as: Y453, Q496, Q498, N501, Y449, Q493, G496, T500, Y505, L455, Q493, and K417; and Mpro: Q189, H164, H163, P168, H41, L167, Q192, M165, C145, Y54, M49, and Q189. Molecular dynamics simulation further established the structural and energetic stability of protein–phytocompound complexes and their interactions with their key residues supporting the molecular docking analysis. Protein–protein docking using ZDOCK and Prodigy server predicted the binding pose and affinity (−13.8 kcal mol−1) of the spike glycoprotein towards the human ACE2 enzyme and also showed significant structural variations in the ACE2 recognition site upon the binding of phyllaemblicin C compound at their binding interface. The phyllaemblicin and cinnamtannin class of phytochemicals can be potential inhibitors of both the spike and Mpro proteins of SARS-CoV-2; furthermore, its pharmacology and clinical optimization would lead towards novel COVID-19 small-molecule therapy.
Phytother Res. 2020 Oct;34(10):2471-2492.
Natural products and their derivatives against coronavirus: A review of the non-clinical and pre-clinical data
Muhammad T Islam 1 2, Chandan Sarkar 3, Dina M El-Kersh 4, Sarmin Jamaddar 3, Shaikh J Uddin 5, Jamil A Shilpi 5, Mohammad S Mubarak 6
- DOI: 10.1002/ptr.6700
Abstract
Several corona viral infections have created serious threats in the last couple of decades claiming the death of thousands of human beings. Recently, corona viral epidemic raised the issue of developing effective antiviral agents at the earliest to prevent further losses. Natural products have always played a crucial role in drug development process against various diseases, which resulted in screening of such agents to combat emergent mutants of corona virus. This review focuses on those natural compounds that showed promising results against corona viruses. Although inhibition of viral replication is often considered as a general mechanism for antiviral activity of most of the natural products, studies have shown that some natural products can interact with key viral proteins that are associated with virulence. In this context, some of the natural products have antiviral activity in the nanomolar concentration (e.g., lycorine, homoharringtonine, silvestrol, ouabain, tylophorine, and 7-methoxycryptopleurine) and could be leads for further drug development on their own or as a template for drug design. In addition, a good number of natural products with anti-corona virus activity are the major constituents of some common dietary supplements, which can be exploited to improve the immunity of the general population in certain epidemics.
Heliyon. 2021 Feb; 7(2): e06350.
Published online 2021 Feb 22. doi: 10.1016/j.heliyon.2021.e06350
Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19
Rajesh K. Thimmulappa,a,∗ Kiran Kumar Mudnakudu-Nagaraju,b Chandan Shivamallu,b K.J.Thirumalai Subramaniam,d Arun Radhakrishnan,d Suresh Bhojraj,c and Gowthamarajan Kuppusamyd,∗
Abstract
Coronavirus disease-19 (COVID-19), a devastating respiratory illness caused by SARS-associated coronavirus-2 (SARS-CoV-2), has already affected over 64 million people and caused 1.48 million deaths, just 12 months from the first diagnosis. COVID-19 patients develop serious complications, including severe pneumonia, acute respiratory distress syndrome (ARDS), and or multiorgan failure due to exaggerated host immune response following infection. Currently, drugs that were effective against SARS-CoV are being repurposed for SARS-CoV-2. During this public health emergency, food nutraceuticals could be promising prophylactic therapeutics for COVID-19. Curcumin, a bioactive compound in turmeric, exerts diverse pharmacological activities and is widely used in foods and traditional medicines. This review presents several lines of evidence, which suggest curcumin as a promising prophylactic, therapeutic candidate for COVID-19. First, curcumin exerts antiviral activity against many types of enveloped viruses, including SARS-CoV-2, by multiple mechanisms: direct interaction with viral membrane proteins; disruption of the viral envelope; inhibition of viral proteases; induce host antiviral responses. Second, curcumin protects from lethal pneumonia and ARDS via targeting NF-κB, inflammasome, IL-6 trans signal, and HMGB1 pathways. Third, curcumin is safe and well-tolerated in both healthy and diseased human subjects. In conclusion, accumulated evidence indicates that curcumin may be a potential prophylactic therapeutic for COVID-19 in the clinic and public health settings.
Coronavirus disease-19 (COVID-19), a devastating respiratory illness caused by SARS-associated coronavirus-2 (SARS-CoV-2), has already affected over 64 million people and caused 1.48 million deaths, just 12 months from the first diagnosis. COVID-19 patients develop serious complications, including severe pneumonia, acute respiratory distress syndrome (ARDS), and or multiorgan failure due to exaggerated host immune response following infection. Currently, drugs that were effective against SARS-CoV are being repurposed for SARS-CoV-2. During this public health emergency, food nutraceuticals could be promising prophylactic therapeutics for COVID-19. Curcumin, a bioactive compound in turmeric, exerts diverse pharmacological activities and is widely used in foods and traditional medicines. This review presents several lines of evidence, which suggest curcumin as a promising prophylactic, therapeutic candidate for COVID-19. First, curcumin exerts antiviral activity against many types of enveloped viruses, including SARS-CoV-2, by multiple mechanisms: direct interaction with viral membrane proteins; disruption of the viral envelope; inhibition of viral proteases; induce host antiviral responses. Second, curcumin protects from lethal pneumonia and ARDS via targeting NF-κB, inflammasome, IL-6 trans signal, and HMGB1 pathways. Third, curcumin is safe and well-tolerated in both healthy and diseased human subjects. In conclusion, accumulated evidence indicates that curcumin may be a potential prophylactic therapeutic for COVID-19 in the clinic and public health settings.
DOI: 10.1039/D0RA03167D (Paper) RSC Adv., 2020, 10, 31385-31399
Curcumin to inhibit binding of spike glycoprotein to ACE2 receptors: computational modelling, simulations, and ADMET studies to explore curcuminoids against novel SARS-CoV-2 targets
Dhivya Shanmugarajana, Prabitha P.a, B. R. Prashantha Kumar*a and B. Sureshb
Abstract
The recent emergence of the novel coronavirus (SARS-CoV-2) has raised global concern as it is declared a pandemic by the WHO. However, to date, there is no current regimen to mitigate the molecular pathogenesis of SARS-CoV-2 virus. Curcuminoids, bioactive ingredients present in Curcuma longa (turmeric), are known to exhibit diverse pharmacological properties. To the best of our understanding to date, SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) for the host cellular entry. This is mediated via proteins of SARS-CoV-2, especially the spike glycoprotein receptor binding domain. Accordingly, our primary objective is to thwart virus replication and binding to the host system, leading us to probe curcuminoids efficiency towards key surface drug target proteins using the computational biology paradigm approach. Specifically, fourteen natural curcuminoids were studied for their possibility of inhibiting SARS-CoV-2. We studied their in silico properties towards SARS-CoV-2 target proteins by homology modelling, ADME, drug-likeness, toxicity predictions, docking molecular dynamics simulations and MM-PBSA free energy estimation. Among the curcuminoids docked to the receptor binding domain of SARS-CoV-2 spike glycoprotein, the keto and enol forms of curcumin form strong hydrogen bond interactions with ACE2 binding residues Q493, T501, Y505, Y489 and Q498. Molecular dynamics simulations, free energy binding and interaction energy validated the interaction and stability of the docked keto and enol forms of curcumin.
J Infect Public Health. 2020 Nov;13(11):1671-1677.
In silico molecular docking: Evaluation of coumarin based derivatives against SARS-CoV-2
Sathish Kumar Chidambaram 1, Daoud Ali 2, Saud Alarifi 2, Surendrakumar Radhakrishnan 1, Idhayadhulla Akbar 3
Abstract
Background: The unique anthropological coronavirus which has been titled as SARS-CoV-2 was originally arisen in late 2019 in Wuhan, China affecting respiratory infection named as COVID-19. Coronavirus is disturbing human life in an exceptional method and has converted a public health global crisis. Natural products are ahead consideration due to the huge beneficial window and effective anti-inflammatory, immunomodulatory, antioxidant and antiviral possessions. Consequently, the present study was intended to display inhibition ability of natural products coumarins and their analogues against SARS coronavirus.
Methods: The present study, aims to forecast theoretical assembly for the protease of COVID-19 and to discover advance whether this protein may assist as a target for protease inhibitors such as psoralen, bergapten, imperatorin, heraclenin, heraclenol, saxalin, oxepeucedanin, angelicin, toddacoumaquinone, and aesculetin. The docking score of these natural coumarin analogues compared with standard Hydroxychloroquine. Whereas the 3D assembly of main protease of SARS coronavirus was forecast with SWISS MODEL web server, and molecular interaction studies amongst target protein and ligands were done with AutoDock Vina software.
Results: The study more exposed that all the inhibitors acquired with negative dock energy against the target protein. Molecular docking investigation displayed that natural coumarin analogue toddacoumaquinone displayed a remarkable inhibition ability with the binding energy of -7.8 kcal/mol than other compounds against main protease of SARS coronavirus in intricate with α-ketoamide (PDB ID: 5N5O). The synthetic coumarin analogue (1 m) also displayed the comparable inhibition ability with a binding energy of -7.1 kcal/mol against main protease of SARS coronavirus in intricate with α-ketoamide. Keeping the overhead results of ADME and toxicity, all tested compounds were recognized as drug-like nature, passing Lipinski’s “Rule of 5” with 0 violation except α-ketoamide passes Lipinski’s “Rule of 5” with 1 violation MW > 500. The projected constraints are within the assortment of recognized values.
Conclusions: Based upon the results of the manifold sequence alliance, natural and synthetic coumarin binding sites were preserved. The present in silico examination thus, delivers structural awareness about the protease of COVID-19 and molecular relations with some of the recognised protease inhibitors.
Front. Chem., 08 February 2021 | https://doi.org/10.3389/fchem.2020.595097
In Silico Study of Coumarins and Quinolines Derivatives as Potent Inhibitors of SARS-CoV-2 Main Protease
Osvaldo Yañez1,2,3, Manuel Isaías Osorio2,4, Eugenio Uriarte5,6, Carlos Areche7, William Tiznado1, José M. Pérez-Donoso2, Olimpo García-Beltrán8* and Fernando González-Nilo2*
Abstract
The pandemic that started in Wuhan (China) in 2019 has caused a large number of deaths, and infected people around the world due to the absence of effective therapy against coronavirus 2 of the severe acute respiratory syndrome (SARS-CoV-2). Viral maturation requires the activity of the main viral protease (Mpro), so its inhibition stops the progress of the disease. To evaluate possible inhibitors, a computational model of the SARS-CoV-2 enzyme Mpro was constructed in complex with 26 synthetic ligands derived from coumarins and quinolines. Analysis of simulations of molecular dynamics and molecular docking of the models show a high affinity for the enzyme (∆Ebinding between −5.1 and 7.1 kcal mol−1). The six compounds with the highest affinity show Kd between 6.26 × 10–6 and 17.2 × 10–6, with binding affinity between −20 and −25 kcal mol−1, with ligand efficiency less than 0.3 associated with possible inhibitory candidates. In addition to the high affinity of these compounds for SARS-CoV-2 Mpro, low toxicity is expected considering the Lipinski, Veber and Pfizer rules. Therefore, this novel study provides candidate inhibitors that would allow experimental studies which can lead to the development of new treatments for SARS-CoV-2.
Antibiotics (Basel). 2021 Apr; 10(4): 420. Published online 2021 Apr 11.
doi: 10.3390/antibiotics10040420
Sinapic Acid Suppresses SARS CoV-2 Replication by Targeting Its Envelope Protein
Raha Orfali,1 Mostafa E. Rateb,2 Hossam M. Hassan,3,4 Mona Alonazi,5 Mokhtar R. Gomaa,6 Noura Mahrous,6 Mohamed GabAllah,6 Ahmed Kandeil,6 Shagufta Perveen,1 Usama Ramadan Abdelmohsen,7,8,* and Ahmed M. Sayed3,*
Abstract
SARS CoV-2 is still considered a global health issue, and its threat keeps growing with the emergence of newly evolved strains. Despite the success in developing some vaccines as a protective measure, finding cost-effective treatments is urgent. Accordingly, we screened a number of phenolic natural compounds for their in vitro anti-SARS CoV-2 activity. We found sinapic acid (SA) selectively inhibited the viral replication in vitro with an half-maximal inhibitory concentration (IC50) value of 2.69 µg/mL with significantly low cytotoxicity (CC50 = 189.3 µg/mL). Subsequently, we virtually screened all currently available molecular targets using a multistep in silico protocol to find out the most probable molecular target that mediates this compound’s antiviral activity. As a result, the viral envelope protein (E-protein) was suggested as the most possible hit for SA. Further in-depth molecular dynamic simulation-based investigation revealed the essential structural features of SA antiviral activity and its binding mode with E-protein. The structural and experimental results presented in this study strongly recommend SA as a promising structural motif for anti-SARS CoV-2 agent development.SARS CoV-2 is still considered a global health issue, and its threat keeps growing with the emergence of newly evolved strains. Despite the success in developing some vaccines as a protective measure, finding cost-effective treatments is urgent. Accordingly, we screened a number of phenolic natural compounds for their in vitro anti-SARS CoV-2 activity. We found sinapic acid (SA) selectively inhibited the viral replication in vitro with an half-maximal inhibitory concentration (IC50) value of 2.69 µg/mL with significantly low cytotoxicity (CC50 = 189.3 µg/mL). Subsequently, we virtually screened all currently available molecular targets using a multistep in silico protocol to find out the most probable molecular target that mediates this compound’s antiviral activity. As a result, the viral envelope protein (E-protein) was suggested as the most possible hit for SA. Further in-depth molecular dynamic simulation-based investigation revealed the essential structural features of SA antiviral activity and its binding mode with E-protein. The structural and experimental results presented in this study strongly recommend SA as a promising structural motif for anti-SARS CoV-2 agent development.
Molecules. 2020 Sep; 25(18): 4103.
Published online 2020 Sep 8. doi: 10.3390/molecules25184103
Polyphenols vs. Coronaviruses: How Far Has Research Moved Forward?
Simona Piccolella, Giuseppina Crescente, Shadab Faramarzi, Marialuisa Formato, Maria Tommasina Pecoraro, and Severina Pacifico*
Abstract
The epidemic, caused by SARS-CoV-2 at the beginning of 2020, led us to a serious change in our lifestyle that for about three months has confined us to our homes, far from our laboratory routine. In this period, the belief that the work of a researcher should never stop has been the driving force in writing the present paper. It aims at reviewing the recent scientific knowledge about in vitro experimental data that focused on the antiviral role of phenols and polyphenols against different species of coronaviruses (CoVs), pointing up the viral targets potentially involved. In the current literature scenario, the papain-like and the 3-chymotrypsin-like proteases seem to be the most deeply investigated and a number of isolated natural (poly)phenols has been screened for their efficacy.
Significant inactivation of SARS-CoV-2 by a green tea catechin, a catechin-derivative and galloylated theaflavins in vitro
Eriko Ohgitani, Masaharu Shin-Ya, Masaki Ichitani, Makoto Kobayashi, Takanobu Takihara, Masaya Kawamoto, Hitoshi Kinugasa, Osam Mazda
doi: https://doi.org/10.1101/2020.12.04.412098
This article is a preprint and has not been certified by peer review [what does this mean?].
Abstract
Potential effects of teas and their constituents on SARS-CoV-2 infection were studied in vitro. Infectivity of SARS-CoV-2 was significantly reduced by a treatment with green tea, roasted green tea or oolong tea. Most remarkably, exposure to black tea for 1 min decreased virus titer to an undetectable level (less than 1/1,000 of untreated control). An addition of (-) epigallocatechin gallate (EGCG) significantly inactivated SARS-CoV-2, while theasinensin A (TSA) and galloylated theaflavins including theaflavin 3, 3’-di-gallate (TFDG) had more remarkable anti-viral activities. Virus treated with TSA at 500 μM or TFDG at 100 μM showed less than 1/10,000 infectivity compared with untreated virus. TSA and TFDG significantly inhibited interaction between recombinant ACE2 and RGD of S protein. These results strongly suggest that EGCG, and more remarkably TSA and galloylated theaflavins, inactivate the novel coronavirus.
Drugs. 2020; 80(14): 1383–1396.
Published online 2020 Jul 23. doi: 10.1007/s40265-020-01365-1
Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19 ?
Vanessa Bianconi,1 Francesco Violi,2 Francesca Fallarino,3 Pasquale Pignatelli,2 Amirhossein Sahebkar,4,5,6 and Matteo Pirro1
Author information Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Abstract
Severe Acute Respiratory Syndrome–Coronavirus-2 is responsible for the current pandemic that has led to more than 10 million confirmed cases of Coronavirus Disease-19 (COVID-19) and over 500,000 deaths worldwide (4 July 2020). Virus-mediated injury to multiple organs, mainly the respiratory tract, activation of immune response with the release of pro-inflammatory cytokines, and overactivation of the coagulation cascade and platelet aggregation leading to micro- and macrovascular thrombosis are the main pathological features of COVID-19. Empirical multidrug therapeutic approaches to treat COVID-19 are currently used with extremely uncertain outcomes, and many others are being tested in clinical trials. Acetylsalicylic acid (ASA) has both anti-inflammatory and antithrombotic effects. In addition, a significant ASA-mediated antiviral activity against DNA and RNA viruses, including different human coronaviruses, has been documented. The use of ASA in patients with different types of infections has been associated with reduced thrombo-inflammation and lower rates of clinical complications and in-hospital mortality. However, safety issues related both to the risk of bleeding and to that of developing rare but serious liver and brain damage mostly among children (i.e., Reye’s syndrome) should be considered. Hence, whether ASA might be a safe and reasonable therapeutic candidate to be tested in clinical trials involving adults with COVID-19 deserves further attention. In this review we provide a critical appraisal of current evidence on the anti-inflammatory, antithrombotic, and antiviral effects of ASA, from both a pre-clinical and a clinical perspective. In addition, the potential benefits and risks of use of ASA have been put in the context of the adult-restricted COVID-19 population.
Severe Acute Respiratory Syndrome–Coronavirus-2 is responsible for the current pandemic that has led to more than 10 million confirmed cases of Coronavirus Disease-19 (COVID-19) and over 500,000 deaths worldwide (4 July 2020). Virus-mediated injury to multiple organs, mainly the respiratory tract, activation of immune response with the release of pro-inflammatory cytokines, and overactivation of the coagulation cascade and platelet aggregation leading to micro- and macrovascular thrombosis are the main pathological features of COVID-19. Empirical multidrug therapeutic approaches to treat COVID-19 are currently used with extremely uncertain outcomes, and many others are being tested in clinical trials. Acetylsalicylic acid (ASA) has both anti-inflammatory and antithrombotic effects. In addition, a significant ASA-mediated antiviral activity against DNA and RNA viruses, including different human coronaviruses, has been documented. The use of ASA in patients with different types of infections has been associated with reduced thrombo-inflammation and lower rates of clinical complications and in-hospital mortality. However, safety issues related both to the risk of bleeding and to that of developing rare but serious liver and brain damage mostly among children (i.e., Reye’s syndrome) should be considered. Hence, whether ASA might be a safe and reasonable therapeutic candidate to be tested in clinical trials involving adults with COVID-19 deserves further attention. In this review we provide a critical appraisal of current evidence on the anti-inflammatory, antithrombotic, and antiviral effects of ASA, from both a pre-clinical and a clinical perspective. In addition, the potential benefits and risks of use of ASA have been put in the context of the adult-restricted COVID-19 population.
Identification of Putative Plant Based Antiviral Compounds to Fight Against SARS-CoV-2 Infection
Mishra SK, Mishra RRR, Dash S, Panigrahi J
Preprint from ChemRxiv, 14 Jul 2020
DOI: 10.26434/chemrxiv.12646613.v1 PPR: PPR187126
Share this article Share with emailShare with twitterShare with linkedinShare with facebook
Abstract
Background: This study aimed to examine the efficacy of some natural compounds and their derivatives in inhibiting the nucleocapsid protein N-terminal RNA binding domain (NSP-NTD), of SARS-CoV-2 virus by using the molecular doacking approach.
Methods: Physiochemical and drug likeness properties of the compounds were characterized by using SWISS ADME server tool. ADMET and TOPKAT modules of Discovery studio 4.0 were used for prediction of pharmacokinetic properties and toxicity of the compounds. Molecular docking of the ligands with the target protein (NSP-NTD) was carried out using the receptor-ligand interactions module of DS 4.0. The CDOCKER energy, CDOCKER interaction energy and binding energy of the interactions were calculated to identify the best interacting compounds.
Results: Four compounds including 4-hydroxybenzoic acid, benzoic acid, 4-aminobenzoic acid and salicylic acid have been predicted as effective compounds to inhibit the NSP-NTD (responsible for packing the viral RNA into the crown like capsid) vis-à-vis combat the SARS-Cov-2 virus infection.
Conclusions: In vitro and in vivo evaluation of these compounds against SARS-CoV-2 virus is required prior to assuring their potential roles in SARS-CoV-2 infection control.
Molecules. 2020 Dec 10;25(24):5828.
Molecular Docking Study on Several Benzoic Acid Derivatives against SARS-CoV-2
Amalia Stefaniu 1, Lucia Pirvu 1, Bujor Albu 1, Lucia Pintilie 1
Abstract
Several derivatives of benzoic acid and semisynthetic alkyl gallates were investigated by an in silico approach to evaluate their potential antiviral activity against SARS-CoV-2 main protease. Molecular docking studies were used to predict their binding affinity and interactions with amino acids residues from the active binding site of SARS-CoV-2 main protease, compared to boceprevir. Deep structural insights and quantum chemical reactivity analysis according to Koopmans’ theorem, as a result of density functional theory (DFT) computations, are reported. Additionally, drug-likeness assessment in terms of Lipinski’s and Weber’s rules for pharmaceutical candidates, is provided. The outcomes of docking and key molecular descriptors and properties were forward analyzed by the statistical approach of principal component analysis (PCA) to identify the degree of their correlation. The obtained results suggest two promising candidates for future drug development to fight against the coronavirus infection.
preprints.org > biology > other > doi: 10.20944/preprints202004.0003.v1
Preprint Article Version 1 Preserved in Portico This version is not peer-reviewed
Computational Screening for Potential Drug Candidates Against SARS-CoV-2 Main Protease
Abstract
Background: SARS-CoV-2 that are the causal agent of a current pandemic are enveloped, positive-sense, single-stranded RNA viruses of the Coronaviridae family. Proteases of SARS-CoV-2 are necessary for viral replication, structural assembly and pathogenicity. The ~33.8KDa Mpro protease of SARS-CoV-2 is a non-human homologue and highly conserved among several coronaviruses indicating Mpro could be a potential drug target for Coronaviruses.Methods: Here we performed computational ligand screening of four pharmacophores (OEW, Remdesivir, Hydroxycholoquine and N3) that are presumed to have positive effects against SARS-CoV-2 Mpro protease (6LU7) and also screened 50,000 molecules from the ZINC Database dataset against this protease target.Results: We found 40 pharmacophore-like structures of natural compounds from diverse chemical classes that exhibited better affinity of docking as compared to the known ligands. The 10 best selected ligands namely, ZINC1845382, ZINC1875405, ZINC2092396, ZINC2104424, ZINC44018332, ZINC2101723, ZINC2094526, ZINC2094304, ZINC2104482, ZINC3984030, and ZINC1531664, are mainly classified as β-carboline, Alkaloids and Polyflavonoids, and all of them displayed interactions with dyad CYS145 and HIS41 from the protease pocket in a similar way as with other known ligands.Conclusion: Our results suggest that these 10 molecules could be effective against SARS-CoV-2 protease and may be tested in vitro and in vivo to develop novel drugs against this virus.
Version 2. F1000Res. 2020; 9: ISCB Comm J-514.
Published online 2020 Dec 21. doi: 10.12688/f1000research.23829.2
PMCID: PMC7780344
Computational screening for potential drug candidates against the SARS-CoV-2 main protease
Bruno Andrade * , Preetam Ghosh , Debmalya Barth , Sandeep Tiwari , Raner José Santana Silva , Wagner Rodrigues de Assis Soares , Tarcisio Silva Melo , Andria dos Santos Freitas , Patrícia González-Grande , Lucas Sousa Palmeira , Luiz Carlos Junior Alcantara , Marta Giovanetti , Aristóteles Góes-Neto , Vasco Ariston de Carvalho Azevedo
Abstract
Background: SARS-CoV-2 is the causal agent of the current coronavirus disease 2019 (COVID-19) pandemic. They are enveloped, positive-sense, single-stranded RNA viruses of the Coronaviridae family. Proteases of SARS-CoV-2 are necessary for viral replication, structural assembly, and pathogenicity. The approximately 33.8 kDa M pro protease of SARS-CoV-2 is a non-human homologue and is highly conserved among several coronaviruses, indicating that M pro could be a potential drug target for Coronaviruses.
Methods: Herein, we performed computational ligand screening of four pharmacophores (OEW, remdesivir, hydroxychloroquine and N3) that are presumed to have positive effects against SARS-CoV-2 M pro protease (6LU7), and also screened 50,000 natural compounds from the ZINC Database dataset against this protease target.
Results: We found 40 pharmacophore-like structures of natural compounds from diverse chemical classes that exhibited better affinity of docking as compared to the known ligands. The 11 best selected ligands, namely ZINC1845382, ZINC1875405, ZINC2092396, ZINC2104424, ZINC44018332, ZINC2101723, ZINC2094526, ZINC2094304, ZINC2104482, ZINC3984030, and ZINC1531664, are mainly classified as beta-carboline, alkaloids, and polyflavonoids, and all displayed interactions with dyad CYS145 and HIS41 from the protease pocket in a similar way as other known ligands.
Conclusions: Our results suggest that these 11 molecules could be effective against SARS-CoV-2 protease and may be subsequently tested in vitro and in vivo to develop novel drugs against this virus.
Background: SARS-CoV-2 is the causal agent of the current coronavirus disease 2019 (COVID-19) pandemic. They are enveloped, positive-sense, single-stranded RNA viruses of the Coronaviridae family. Proteases of SARS-CoV-2 are necessary for viral replication, structural assembly, and pathogenicity. The approximately 33.8 kDa M pro protease of SARS-CoV-2 is a non-human homologue and is highly conserved among several coronaviruses, indicating that M pro could be a potential drug target for Coronaviruses.
J Gen Virol. 2021 Apr;102(4).
The green tea catechin epigallocatechin gallate inhibits SARS-CoV-2 infection
Lisa Henss 1, Arne Auste 2 3, Christoph Schürmann 3, Christin Schmidt 1, Christine von Rhein 1, Michael D Mühlebach 2 3, Barbara S Schnierle 1
- DOI: 10.1099/jgv.0.001574
Abstract
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection has caused a pandemic with tens of millions of cases and more than a million deaths. The infection causes COVID-19, a disease of the respiratory system of divergent severity. No treatment exists. Epigallocatechin-3-gallate (EGCG), the major component of green tea, has several beneficial properties, including antiviral activities. Therefore, we examined whether EGCG has antiviral activity against SARS-CoV-2. EGCG blocked not only the entry of SARS-CoV-2, but also MERS- and SARS-CoV pseudotyped lentiviral vectors and inhibited virus infections in vitro. Mechanistically, inhibition of the SARS-CoV-2 spike-receptor interaction was observed. Thus, EGCG might be suitable for use as a lead structure to develop more effective anti-COVID-19 drugs.
Methods: Herein, we performed computational ligand screening of four pharmacophores (OEW, remdesivir, hydroxychloroquine and N3) that are presumed to have positive effects against SARS-CoV-2 M pro protease (6LU7), and also screened 50,000 natural compounds from the ZINC Database dataset against this protease target.
Results: We found 40 pharmacophore-like structures of natural compounds from diverse chemical classes that exhibited better affinity of docking as compared to the known ligands. The 11 best selected ligands, namely ZINC1845382, ZINC1875405, ZINC2092396, ZINC2104424, ZINC44018332, ZINC2101723, ZINC2094526, ZINC2094304, ZINC2104482, ZINC3984030, and ZINC1531664, are mainly classified as beta-carboline, alkaloids, and polyflavonoids, and all displayed interactions with dyad CYS145 and HIS41 from the protease pocket in a similar way as other known ligands.
Conclusions: Our results suggest that these 11 molecules could be effective against SARS-CoV-2 protease and may be subsequently tested in vitro and in vivo to develop novel drugs against this virus.
Severe Acute Respiratory Syndrome–Coronavirus-2 is responsible for the current pandemic that has led to more than 10 million confirmed cases of Coronavirus Disease-19 (COVID-19) and over 500,000 deaths worldwide (4 July 2020). Virus-mediated injury to multiple organs, mainly the respiratory tract, activation of immune response with the release of pro-inflammatory cytokines, and overactivation of the coagulation cascade and platelet aggregation leading to micro- and macrovascular thrombosis are the main pathological features of COVID-19. Empirical multidrug therapeutic approaches to treat COVID-19 are currently used with extremely uncertain outcomes, and many others are being tested in clinical trials. Acetylsalicylic acid (ASA) has both anti-inflammatory and antithrombotic effects. In addition, a significant ASA-mediated antiviral activity against DNA and RNA viruses, including different human coronaviruses, has been documented. The use of ASA in patients with different types of infections has been associated with reduced thrombo-inflammation and lower rates of clinical complications and in-hospital mortality. However, safety issues related both to the risk of bleeding and to that of developing rare but serious liver and brain damage mostly among children (i.e., Reye’s syndrome) should be considered. Hence, whether ASA might be a safe and reasonable therapeutic candidate to be tested in clinical trials involving adults with COVID-19 deserves further attention. In this review we provide a critical appraisal of current evidence on the anti-inflammatory, antithrombotic, and antiviral effects of ASA, from both a pre-clinical and a clinical perspective. In addition, the potential benefits and risks of use of ASA have been put in the context of the adult-restricted COVID-19 population.
Severe Acute Respiratory Syndrome–Coronavirus-2 is responsible for the current pandemic that has led to more than 10 million confirmed cases of Coronavirus Disease-19 (COVID-19) and over 500,000 deaths worldwide (4 July 2020). Virus-mediated injury to multiple organs, mainly the respiratory tract, activation of immune response with the release of pro-inflammatory cytokines, and overactivation of the coagulation cascade and platelet aggregation leading to micro- and macrovascular thrombosis are the main pathological features of COVID-19. Empirical multidrug therapeutic approaches to treat COVID-19 are currently used with extremely uncertain outcomes, and many others are being tested in clinical trials. Acetylsalicylic acid (ASA) has both anti-inflammatory and antithrombotic effects. In addition, a significant ASA-mediated antiviral activity against DNA and RNA viruses, including different human coronaviruses, has been documented. The use of ASA in patients with different types of infections has been associated with reduced thrombo-inflammation and lower rates of clinical complications and in-hospital mortality. However, safety issues related both to the risk of bleeding and to that of developing rare but serious liver and brain damage mostly among children (i.e., Reye’s syndrome) should be considered. Hence, whether ASA might be a safe and reasonable therapeutic candidate to be tested in clinical trials involving adults with COVID-19 deserves further attention. In this review we provide a critical appraisal of current evidence on the anti-inflammatory, antithrombotic, and antiviral effects of ASA, from both a pre-clinical and a clinical perspective. In addition, the potential benefits and risks of use of ASA have been put in the context of the adult-restricted COVID-19 population.
Severe Acute Respiratory Syndrome–Coronavirus-2 is responsible for the current pandemic that has led to more than 10 million confirmed cases of Coronavirus Disease-19 (COVID-19) and over 500,000 deaths worldwide (4 July 2020). Virus-mediated injury to multiple organs, mainly the respiratory tract, activation of immune response with the release of pro-inflammatory cytokines, and overactivation of the coagulation cascade and platelet aggregation leading to micro- and macrovascular thrombosis are the main pathological features of COVID-19. Empirical multidrug therapeutic approaches to treat COVID-19 are currently used with extremely uncertain outcomes, and many others are being tested in clinical trials. Acetylsalicylic acid (ASA) has both anti-inflammatory and antithrombotic effects. In addition, a significant ASA-mediated antiviral activity against DNA and RNA viruses, including different human coronaviruses, has been documented. The use of ASA in patients with different types of infections has been associated with reduced thrombo-inflammation and lower rates of clinical complications and in-hospital mortality. However, safety issues related both to the risk of bleeding and to that of developing rare but serious liver and brain damage mostly among children (i.e., Reye’s syndrome) should be considered. Hence, whether ASA might be a safe and reasonable therapeutic candidate to be tested in clinical trials involving adults with COVID-19 deserves further attention. In this review we provide a critical appraisal of current evidence on the anti-inflammatory, antithrombotic, and antiviral effects of ASA, from both a pre-clinical and a clinical perspective. In addition, the potential benefits and risks of use of ASA have been put in the context of the adult-restricted COVID-19 population.
Epigallocatechin Gallate from Green Tea Effectively Blocks Infection of SARS-CoV-2 and New Variants by Inhibiting Spike Binding to ACE2 Receptor
Jinbiao Liu, Brittany H Bodnar, Fengzhen Meng, Adil Khan, Xu Wang, Guangxiang Luo, Sami Saribas, Tao Wang, Saroj Chandra Lohani, Peng Wang, Zhengyu Wei, Jinjun Luo, Lina Zhou, Jianguo Wu, Qingsheng Li, Wenhui Hu, Wenzhe Ho
doi: https://doi.org/10.1101/2021.03.17.435637
This article is a preprint and has not been certified by peer review [what does this mean?].
Abstract
As the COVID-19 pandemic rages on, the new SARS-CoV-2 variants have emerged in the different regions of the world. These newly emerged variants have mutations in their spike (S) protein that may confer resistance to vaccine-elicited immunity and existing neutralizing antibody therapeutics. Therefore, there is still an urgent need of safe, effective, and affordable agents for prevention/treatment of SARS-CoV-2 and its variant infection. Here, we demonstrated that green tea beverage (GTB) or its major ingredient, epigallocatechin gallate (EGCG), were highly effective in inhibiting infection of live SARS-CoV-2 and human coronavirus (HCoV OC43). In addition, infection of the pseudoviruses with spikes of the new variants (UK-B.1.1.7, SA-B.1.351, and CA-B.1.429) was efficiently blocked by GTB or EGCG. Among the 4 active green tea catechins at noncytotoxic doses, EGCG was the most potent in the action against the viruses. The highest inhibitory activity was observed when the viruses or the cells were pre-incubated with EGCG prior to the infection. Mechanistic studies revealed that EGCG blocked infection at the entry step through interfering with the engagement of the receptor binding domain (RBD) of the viral spikes to angiotensin-converting enzyme 2 (ACE2) receptor of the host cells. These data support further clinical evaluation and development of EGCG as a novel, safe, and cost-effective natural product for prevention/treatment of SARS-CoV-2 transmission and infection.
Molecules. 2020 Sep; 25(18): 4125.
Published online 2020 Sep 9. doi: 10.3390/molecules25184125
Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications
Adam Kowalczyk,1 Martyna Przychodna,2 Sylwia Sopata,2 Agnieszka Bodalska,1,* and Izabela Fecka1
Abstract
Thymol (2-isopropyl-5-methylphenol) belongs to the phenolic monoterpenes and mostly occurs in thyme species. It is one of the main compounds of thyme essential oil. Both thymol and thyme essential oil have long been used in traditional medicine as expectorant, anti-inflammatory, antiviral, antibacterial, and antiseptic agents, mainly in the treatment of the upper respiratory system. The current search for new directions of biological or therapeutic activities of natural plant substances with known structures includes thyme essential oil and thymol. Novel studies have demonstrated their antibiofilm, antifungal, antileishmanial, antiviral, and anticancer properties. Also, their new therapeutic formulations, such as nanocapsules containing these constituents, can be beneficial in medicinal practice and create opportunities for their extensive use. Extensive application of thymol and thyme essential oil in the healthcare sector is very promising but requires further research and analysis.Thymol (2-isopropyl-5-methylphenol) belongs to the phenolic monoterpenes and mostly occurs in thyme species. It is one of the main compounds of thyme essential oil. Both thymol and thyme essential oil have long been used in traditional medicine as expectorant, anti-inflammatory, antiviral, antibacterial, and antiseptic agents, mainly in the treatment of the upper respiratory system. The current search for new directions of biological or therapeutic activities of natural plant substances with known structures includes thyme essential oil and thymol. Novel studies have demonstrated their antibiofilm, antifungal, antileishmanial, antiviral, and anticancer properties. Also, their new therapeutic formulations, such as nanocapsules containing these constituents, can be beneficial in medicinal practice and create opportunities for their extensive use. Extensive application of thymol and thyme essential oil in the healthcare sector is very promising but requires further research and analysis.
Front. Chem., 29 March 2021 | https://doi.org/10.3389/fchem.2021.642026
Antiviral Essential Oil Components Against SARS-CoV-2 in Pre-procedural Mouth Rinses for Dental Settings During COVID-19: A Computational Study
Pradeep Kumar Yadalam1, Kalaivani Varatharajan2, K. Rajapandian2, Priyanka Chopra3, Deepavalli Arumuganainar4, Thilgavathi Nagarathnam1, Honglae Sohn5* and Thirumurthy Madhavan6*
COVID-19 mainly spreads through cough or sneeze droplets produced by an infected person. The viral particles are mostly present in the oral cavity. The risk of contracting COVID-19 is high in the dental profession due to the nature of procedures involved that produce aerosols. Along with other measures to limit the risk of infection, pre-procedural mouth rinses are beneficial in reducing the viral particles in the oral cavity. In this study, the antiviral efficacy of essential oil components has been determined specifically against SARS-CoV-2 by molecular docking and conceptual DFT approach. Based on the binding affinities of the components against the receptor binding domain of the S1 glycoprotein, cuminal, carvacrol, myrtanol, and pinocarveol were found to be highly active. The molecular descriptor values obtained through conceptual DFT also indicated the above-mentioned components to be active based on the correlation between the structure and the activity of the compounds. Therefore, pre-procedural mouth rinses with these components included may be specifically suitable for dental procedures during the COVID-19 period.
Introduction
The outbreak of corona virus disease 2019 (COVID-19) in Wuhan, China, has impacted the world in several ways (Lai et al., 2020). This disease, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has swiftly spread across 202 countries in the world due to its highly contagious nature (Peng et al., 2020b). As per the World Health Organization (WHO) report, there have been about 38 million confirmed cases of COVID-19, including one million deaths all over the world (as on October 16, 2020) (https://covid19.who.int/). And in India alone, there are seven million cases with about 100,000 deaths reported (as on October 12, 2020) (WHO Coronavirus Disease, 2020). Despite undertaking serious measures to contain the disease globally, it is still on the rise with no vaccine or drug to control the same. The virus spreads through direct contact with cough and sneeze droplets from an infected person or by touching contaminated surfaces and further by touching the nose or mouth (Dhand and Li, 2020). Once a person contracts the disease, the viral particles are mostly housed in the nasal cavity, oropharynx, nasopharynx, and salivary secretions (Han and Ivanovski, 2020; Krajewska Wojciechowska et al., 2020). An infected person displays symptoms such as fever, cough, and cold, a there have been reports indicating that asymptomatic carriers also spread the disease (Qu et al., 2020; Yu and Yang, 2020).
The nature of dental doctors’ work mostly involves being in close proximity with patients and exposure to saliva and blood from aerosols generated from regular dental procedures, which puts them at high risk of viral infection (Li et al., 2020; Meng et al., 2020; Peng et al., 2020a). The droplets may infect the dentist if they are large in size; otherwise, they may remain suspended in the air and cause long-distance transmission in case of smaller droplets (Baghizadeh Fini, 2020). Several studies suggest that SARS-CoV-2 spike protein (1273 amino acid residues) binds to human angiotensin converting enzyme 2 (ACE-2) and utilizes it as a cellular entry receptor for binding and replication (Gurwitz, 2020; Verdecchia et al., 2020; Ziegler et al., 2020). The spike (S) protein is composed of two subunits, namely, S1 and S2. The receptor binding domain (RBD) of the S1 protein (319–541 residues) binds to the ACE-2 cell receptor, followed by fusion, which involves the S2 protein. The RBD lies in the C-terminal domain of the S1 protein, which has more residues that directly interact with the ACE-2 receptor when compared to the N-terminal domain (Huang et al., 2020). The domains of S glycoprotein and structure of SARS-CoV-2 are depicted in Figure 1. Hence, this region is a critical target for antibodies or antiviral compounds. ACE-2 receptors are abundantly present in the salivary glands and lungs (Xu et al., 2020). Therefore, dental professionals must exercise extreme care in terms of safety to prevent nosocomial infection. Dental societies and associations have laid down guidelines to control the transmission of the disease by suggesting dental professionals either completely stop providing dental services or postpone elective treatments and provide primary care through telemedicine services. Only emergency treatments are permitted to be performed by wearing personal protective equipment (PPE) and treating the patients with pre-procedural mouth rinse (PPMR) as a precaution to avoid any possible infection (Jevon and Shamsi, 2020; Nimbulkar et al., 2020). Recent studies have acknowledged the effectiveness of PPMR components such as povidone-iodine, 0.12%-chlorhexidine gluconate, cetylpyridinium chloride, chloroxylenol, benzalkonium chloride, and cetrimide/chlorhexidine in dental care to limit the viral load prior to treatment (Herrera et al., 2020; Meng et al., 2020). Certain essential oil (EO) components such as menthol, thymol, eugenol, and eucalyptol are common active ingredients in mouth rinses (Vlachojannis et al., 2013; Alshehri, 2018). Essential oils are a complex mixture of aromatic compounds that are known for antimicrobial activity against a host of microbes (Bakkali et al., 2008). The activity of these compounds is mostly related to their structure. Previously, numerous studies have proven the efficacy of EOs against many viruses such as herpes simplex virus (type 1 and type 2), influenza virus adenovirus type 3, and poliovirus (Minami et al., 2003; Koch et al., 2008; Swamy et al., 2016; Tariq et al., 2019). The study of synergistic activity among the EO components may lead to better antimicrobial activity. The main advantage of using EOs for therapy, against synthetic drugs, is that they fall under the GRAS (generally regarded as safe) category, whereas synthetic drugs have to undergo various levels of safety and toxicity testing, which is time-consuming. EOs are generally used for therapeutic benefits in complementary and alternative medicine (CAM) to treat infectious, non-infectious, and psychological disorders. Hence, in this study, we aim to identify EO components that are comparable or better in terms of activity, in comparison with the ones that are commonly used.
Journal of Molecular Structure
Volume 1221, 5 December 2020, 128823
Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein
Seema A.KulkarniaSanthosh KumarNagarajanbVeenaRameshcVelusamyPalaniyandiaS. PeriyarSelvamdThirumurthyMadhavanb
https://doi.org/10.1016/j.molstruc.2020.128823Get rights and content
Highlights
Essential oils components as promising candidates for wide range of therapeutic activities.
Residues in the distal end of RBD of spike protein as a key region for targeting novel antivirals.
Identification of potential antiviral compounds from essential oils through molecular docking and DFT approach.
Anethole, Cinnamaldehyde, Carvacrol exhibit better inhibition of RBD region.
Abstract
COVID-19, caused by SARS-CoV-2 has recently emerged as a global pandemic. Intense efforts are ongoing to find a vaccine or a drug to control the disease across the globe. Meanwhile, alternative therapies are also being explored to manage the disease. Phytochemicals present in essential oils are promising candidates which have been known to possess wide range of therapeutic activities. In this study, major components of several essential oils which are known for their antimicrobial properties have been docked against the S1 receptor binding domain of the spike (S) glycoprotein, which is the key target for novel antiviral drugs, to ascertain their inhibitory effects based on their binding affinities. It has been found that some monoterpenes, terpenoid phenols and phenyl propanoids such as anethole, cinnamaldehyde, carvacrol, geraniol, cinnamyl acetate, L-4-terpineol, thymol and pulegone from essential oils extracted from plants belonging to families such as Lamiaceae, Lauraceae, Myrtaceae, Apiaceae, Geraniaceae and Fabaceae are effective antiviral agents that have potential to inhibit the viral spikeprotein.
In vitro screening of anti-viral and virucidal effects against SARS-CoV-2 by Hypericum perforatum and Echinacea
Leena Hussein Bajrai, Sherif Ali El-Kafrawy, Rabie Saleh Alnahas, Esam Ibraheem Azhar
doi: https://doi.org/10.1101/2021.01.11.426295
This article is a preprint and has not been certified by peer review [what does this mean?].
Abstract
Special Infectious Agent Unit in King Fahd Medical Research Center at King Abdulaziz University, Jeddah, Saudi Arabia, has pursed the anti-viral project field to optimize the group of medicinal plants for human-infectious diseases. We have begun virtually in this field since COVID-19 pandemic, besides our divergence in the infectious agents’. In this study and based on the previous review, Hypericum perforatum (St. John’s Wort) and Echinacea (gaia HERBS®) were tested in vitro using Vero E6 cells for their anti-viral effects against the newly identified Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) through its infectious cycle from 0 to 48 hours post infection. The hypericin (0.9 mg) of H. perforatum and the different parts (roots, seeds, aerial) of two types of Echinacea species (Echinacea purpurea and Echinacea angustifolia) were examined their efficacy in certain concentration and under light-dependent anti-viral activities to measure the inhibition of the SARS-CoV-2 mRNA expression of RNA-dependent RNA polymerase (RdRP) gene and the viral load with quantitative real-time polymerase chain reaction (qRT-PCR), and to assess the neutralization of the SARS-CoV-2 spike receptor binding on cell culture assay. Interestingly, the mixture (H.E.) of 100 mg/mL of H. perforatum and Echinacea was tested too on SARS-CoV-2 and showed crucial anti-viral activity competing H. perforatum then Echinacea effects as anti-viral treatment. Therefore, the results of gaia HERBS® products, H. perforatum and Echinacea species, applied in this study showed significant anti-viral and virucidal effects in the following order of potency: H. perforatum, H.E., and Echinacea on SARS-CoV-2 infectious cycle; and will definitely required a set up of clinical trial with specific therapeutic protocol based on the outcome of this study.
Informatics in Medicine Unlocked
Volume 20, 2020, 100407
Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors
NegarBalmehaSamiraMahmoudibNiloofarMohammadicAnasikKarabedianhajiabadid
Show more
https://doi.org/10.1016/j.imu.2020.100407Get rights and content
Highlights
Different therapeutic targets were found for inhibiting SARS-CoV-2.
Molecular docking analysis of medicinal herbal compounds were conducted with SARS-CoV-2 receptors.
The affinity of different miRNAs to 3′ UTR of SARS-CoV-2 genome were investigated.
The role of miRNAs in the human cell signaling pathways in which SARS-CoV-2 is involved was investigated.
Abstract
The SARS-CoV-2 causes severe pulmonary infectious disease with an exponential spread-ability. In the present research, we have tried to look into the molecular cause of disease, dealing with the development and spread of the coronavirus disease 2019 (COVID-19). Therefore, different approaches have investigated against disease development and infection in this research; First, We identified hsa-miR-1307-3p out of 1872 pooled microRNAs, as the best miRNA, with the highest affinity to SARS-CoV-2 genome and its related cell signaling pathways. Second, the findings presented that this miRNA had a considerable role in PI3K/Act, endocytosis, and type 2 diabetes, moreover, it may play a critical role in the prevention of GRP78 production and the virus entering, proliferation and development. Third, nearly 1033 medicinal herbal compounds were collected and docked with ACE2, TMPRSS2, GRP78, and AT1R receptors, which were the most noticeable receptors in causing the COVID-19. Among them, there were three common compounds including berbamine, hypericin, and hesperidin, which were more effective and appropriate to prevent the COVID-19 infection. Also, it was revealed some of these chemical compounds which had a greater affinity for AT1R receptor inhibitors can be suitable therapeutic targets for inhibiting AT1R and preventing the adverse side effects of this receptor. According to the result, clinical assessment of these three herbal compounds and hsa-miR-1307-3p may have significant outcomes for the prevention, control, and treatment of COVID-19 infection.
J Med Chem. 2007 Aug 23;50(17):4087-95.
Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus
Chih-Chun Wen 1, Yueh-Hsiung Kuo, Jia-Tsrong Jan, Po-Huang Liang, Sheng-Yang Wang, Hong-Gi Liu, Ching-Kuo Lee, Shang-Tzen Chang, Chih-Jung Kuo, Shoei-Sheng Lee, Chia-Chung Hou, Pei-Wen Hsiao, Shih-Chang Chien, Lie-Fen Shyur, Ning-Sun Yang
- DOI: 10.1021/jm070295s
Abstract
In this study, 221 phytocompounds were evaluated for activity against anti-severe acute respiratory syndrome associated coronavirus (SARS-CoV) activities using a cell-based assay measuring SARS-CoV-induced cytopathogenic effect on Vero E6 cells. Ten diterpenoids (1-10), two sesquiterpenoids (11 and 12), two triterpenoids (13 and 14), five lignoids (15-19), curcumin (20), and reference controls niclosamide (21) and valinomycin (22) were potent inhibitors at concentrations between 3.3 and 10 microM. The concentrations of the 22 compounds to inhibit 50% of Vero E6 cell proliferation (CC50) and viral replication (EC50) were measured. The selective index values (SI = CC50/EC50) of the most potent compounds 1, 5, 6, 8, 14, and 16 were 58, >510, 111, 193, 180, and >667, respectively. Betulinic acid (13) and savinin (16) were competitive inhibitors of SARS-CoV 3CL protease with Ki values = 8.2 +/- 0.7 and 9.1 +/- 2.4 microM, respectively. Our findings suggest that specific abietane-type diterpenoids and lignoids exhibit strong anti-SARS-CoV effects.
In Silico Identification of Potential Allosteric Inhibitors of the SARS-CoV-2 Helicase
Download (1.4 MB)Export as PDF
Preprint
revised on 29.06.2020, 14:30 and posted on 30.06.2020, 13:00 by Adekunle Rowaiye, Olukemi Onuh, Titilayo Asala, Amoge Ogu, Doofan Bur, Ezinne Nwankwo, Uchenna Orji, Zainab Ibrahim, Jamila Hamza, Adaku Ugorji
The COVID-19 pandemic ravages the globe causing unprecedented health and economic challenges. As the world prospects for a cure, scientists are looking critically at strategic protein targets within the SARS-CoV-2 that have therapeutic significance. One of such targets is the Helicase which is an enzyme that affects all aspects of SARS-CoV-2 RNA metabolism. The aim of this study is to identify small molecules from natural products that have strong binding affinity with and inhibitory activity against an allosteric site (Pocket 26) of SARS-CoV-2 Helicase. Pyrx was used for the in silico molecular docking simulations of SARS-CoV-2 Helicase (QHD43415-12.pdb) against a library of small molecules obtained from edible African plants. Triphenylmethane which had a docking score of -7.4 kcal/mol was chosen as a reference molecule. Virtual screening for oral bioavailability was done based on the molecular descriptors of the compounds as provided by Pubchem. SwissADME, pkCSM, and Molinspiration were used for further screening for molar refractivity, saturation, promiscuity, pharmacokinetic properties, and bioactivity respectively. The Galaxy webserver which uses the GROMACS software was used for the molecular dynamic simulation and analyses. The lead compounds are Gibberellin A12, A20 and A51 obtained from Green peas and the Okra plant. Gibberellin A20 and A51 performed better than the standard. Gibberellin A51 is predicted to show the greatest inhibitory activity against SARS-CoV-2 Helicase. It is recommended that the inhibitory activities of the lead compounds be further investigated.
In vitro dual (anticancer and antiviral) activity of the carotenoids produced by haloalkaliphilic archaeon Natrialba sp. M6
Ghada E. Hegazy, Marwa M. Abu-Serie, Gehan M. Abo-Elela, Hanan Ghozlan, Soraya A. Sabry, Nadia A. Soliman & Yasser R. Abdel-Fattah
Scientific Reports volume 10, Article number: 5986 (2020) Cite this article
Abstract
Halophilic archaea are a promising natural source of carotenoids. However, little information is available about the biological impacts of these archaeal metabolites. Here, carotenoids of Natrialba sp. M6, which was isolated from Wadi El-Natrun, were produced, purified and identified by Raman spectroscopy, GC-mass spectrometry, and Fourier transform infrared spectroscopy, LC–mass spectrometry and Nuclear magnetic resonance spectroscopy. The C50 carotenoid bacterioruberin was found to be the predominant compound. Because cancer and viral hepatitis are serious diseases, the anticancer, anti-HCV and anti-HBV potentials of these extracted carotenoids (pigments) were examined for the first time. In vitro results indicated that the caspase-mediated apoptotic anticancer effect of this pigment and its inhibitory efficacy against matrix metalloprotease 9 were significantly higher than those of 5-fluorouracil. Furthermore, the extracted pigment exhibited significantly stronger activity for eliminating HCV and HBV in infected human blood mononuclear cells than currently used drugs. This antiviral activity may be attributed to its inhibitory potential against HCV RNA and HBV DNA polymerases, which thereby suppresses HCV and HBV replication, as indicated by a high viral clearance % in the treated cells. These novel findings suggest that the C50 carotenoid of Natrialba sp. M6 can be used as an alternative source of natural metabolites that confer potent anticancer and antiviral activities.
Introduction
Halophilic archaea (haloarchaea) belong to the family Halobacteriaceae. This family includes a group of microorganisms that are able to live in hypersaline environments with high salt concentrations (up to 4 M), such as solar salterns, salt lakes and salt deposits1. Interestingly, these organisms have received increasing attention due to their ability to produce a plethora of compounds with potential applications in many fields of biotechnology, including salt-tolerant enzymes, biodegradable polyesters, exopolysaccharides, antimicrobial halocins, biosurfactants, and photon-driven retinal protein2. Most haloarchaeal species (e.g., Natrialba) can produce pigments, including carotenoids. Natrialba is an organism in our research that belongs to the Halobacteriaceae family. However, the genus has recently been reassigned to the novel family Natrialbaceae3. Natrialba sp. M6 is an extreme haloalkaliphile that grows at pH 10.0 and 20–25% w/v NaCl and utilizes a wide range of carbohydrate and noncarbohydrate substrates.
Carotenoids are lipid-soluble pigments that vary in colour between yellow, orange, and red. Carotenoids are classified based on the number of carbons in their backbones into the categories C30, C40 and C50. Most carotenoids exist as a C40 structure in different living organisms, including bacteria, archaea, fungi, algae, and plants4. Meanwhile, haloarchaea can produce C50 bacterioruberin (BR), a rare carotenoid form that contains four hydroxyl groups5. These pigments are divided into two major groups: xanthophylls (molecules containing oxygen), such as lutein and zeaxanthin, and carotenes (nonoxygenated molecules), such as α-carotene and lycopene6. The presence of carotenoids in the membrane of archaeal cells may help cells adapt to hypersaline environments by acting as a water barrier and allowing ions and oxygen molecules to permeate through the cell membrane. Additionally, BR protects bacteria from damage under intensive light and against free radical- and UV-mediated oxidative DNA damage7. Therefore, these carotenoids can stabilize archaeal cells under high osmotic and oxidative stresses8,9. The biological function of carotenoids is mostly attributed to their antioxidant properties that protect cells from oxidative damage; consequently, they improve human health10.
However, haloarchaeal carotenoids were first studied in the 1960s11. Few studies during the last half of the 20th century focused on haloarchaeal carotenoids compared with carotenoids of other organisms. In addition, little information on the biological properties of haloarchaeal carotenoids is available, and there is an increasing need for new sources of such important bioactive compounds. Hence, after partial extraction of carotenoids produced by the haloalkaliphilic archaeon Natrialba sp. M6, we evaluated their in vitro anticancer activity by investigating their potential for the induction of apoptosis-dependent cell death and for blocking matrix metalloprotease (MMP) 9-dependent cancer progression (angiogenesis and metastasis)12. Additionally, we investigated the antiviral activity of these carotenoids by assessing their impact on hepatitis C virus (HCV) RNA-dependent RNA and hepatitis B virus (HBV) DNA-dependent DNA polymerase-mediated viral replication. This evaluation will open the door for more research on the possible therapeutic applications of carotenoids as natural remedies.
In vitro antiviral activity of lutein against hepatitis B virus
Ran Pang , Jun‐Yan Tao, Shu‐Ling Zhang, Lei Zhao, Xin Yue, Yue‐Feng Wang, Pian Ye, Ji‐Hua Dong, Ying Zhu, Jian‐Guo Wu
https://doi.org/10.1002/ptr.3155
Abstract
Despite the availability of an effective vaccine, the hepatitis B virus (HBV) infection and its treatment remains one of the foremost public health problems in the world. The present study was performed in order to investigate the anti‐HBV activity of lutein in vitro. The antiviral activity of lutein was examined by detecting the levels of HBsAg, HBeAg and extracellular HBV DNA in stable HBV‐producing human hepatoblastoma HepG2 2.2.15 cells. It was found that lutein effectively suppressed the secretion of HBsAg from HepG2 2.2.15 cells in a dose‐dependent manner, and it also suppressed the amount of extracellular HBV DNA. A luciferase reporter gene assay was used to determine the effects of lutein on the activities of HBV promoters. The results showed that lutein inhibited the activity of HBV full‐length promoter (Fp). These data indicate that lutein possesses an anti‐HBV activity and exerts its antivirus effects via inhibition of HBV transcription. Copyright © 2010 John Wiley & Sons, Ltd.
Natural products’ role against COVID-19
Ananda da Silva Antonio, a Larissa Silveira Moreira Wiedemann a and Valdir Florêncio Veiga-Junior *ab
https://pubs.rsc.org/en/content/articlelanding/2020/ra/d0ra03774e#!divAbstract
Abstract
COVID-19 is a viral disease caused by a new severe acute respiratory syndrome (SARS-CoV-2), which has quickly resulted in a pandemic. As a great threat to global public health, the development of a treatment has become vital, and a rush to find a cure has mobilized researchers from all areas across the world. Synthetic drugs, such as hydroxychloroquine, have gained attention. However, the efficacy of repositioned drugs is still under evaluation, and besides, some severe side effects are a cause for concern. This emphasizes the urgency for treatment options, which can be both safe and effective. With this in mind, natural products could be an important resource in the development of COVID-19 treatment, as they have already contributed in the past to treatments against other viruses, such as HIV, MERS-CoV, and influenza. Natural products are described long term as bioactive substances and some phytochemical classes such as flavonoids, alkaloids, and peptides are known antiviral bioproducts, and have been virtually tested with success against COVID-19. However, important issues still need to be addressed as to their bioavailability and true efficacy in vivo. This review intends to systematically evaluate the natural metabolites that could potentially be used against this new disease looking at their natural sources, mechanism of action and previous pharmacological usages. The aim is to provide a starting point for this research area in order to speed up the establishment of anti-SARS-CoV-2 bioproducts.
Therapeutic Potential of Calendula officinalis
Vrish Dhwaj Ashwlayan, Amrish Kumar, Mansi Verma, Vipin Kumar Garg, SK Gupta
Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, India
Correspondence: Vrish Dhwaj Ashwlayan, Department of Pharmaceutical Technology, Meerut Institute of Engineering and Technology, India
Received: January 20, 2018 | Published: April 20, 2018
Citation: AshwlayanVD, Kumar A, Verma M, et al. Therapeutic Potential of Calendula officinalis. Pharm Pharmacol Int J. 2018;6(2):149-155.
DOI: 10.15406/ppij.2018.06.00171
Abstract
Calendula officinalis(Calendula), belonging to the family of Asteraceae, commonly known as English Marigold or Pot Marigold is an aromatic herb which is used in Traditional system of medicine for treating wounds, ulcers, herpes, scars, skin damage, frost-bite and blood purification. It is mainly used because of its various biological activities to treat diseases like analgesic, anti–diabetic, anti-ulcer and anti-inflammatory. It is also used for in gastro-intestinal, gynecological, eye disease, skin injuries and in some cases of burn. Calendula oil is still medicinally used as, an anti-tumor agent, and a remedy for healing wounds. Plant pharmacological studies have suggested that Calendula extracts have antiviral, anti-genotoxic properties in-vitro. In herbalism, Calendula in suspension or in tincture is used topically for treating acne, reducing inflammation, controlling bleeding, and soothing irritated tissue. Calendula is used for protection against the plague. In early American Shaker medicine, calendula was a treatment for gangrene. In addition to its first aid uses, calendula also acts as a digestive remedy. An infusion or tincture of the flowers, taken internally, is beneficial in the treatment of yeast infections, and diarrhea. An infusion of Calendula officinalismay also be used to treating bee stings, eye inflammations, boils and abscesses, varicose veins, eczema, and as a gargle for mouth sores or to relieve toothache. It improves the circulation of the blood & the lymphatic fluids and aids in elimination of toxins from the body. This plant is rich in many pharmaceutical active ingredients like carotenoids, flavonoids, glycosides, steroids and sterols quinines, volatile oil, and amino acid. The extract of this plant as well as pure compound isolated from it, have been demonstrated to possess multiple pharmacological activities such as anti-cytotoxic, hepato-protective and spasmolytic amongst others. Acute toxicity studies in rats and mice suggest that the extract is relatively nontoxic. Animal tests have demonstrated minimal skin irritation, and no sensitization or photo toxicity. Minimal ocular irritation was seen with one formulation and no irritation with others. Six saponins isolated from C. officinalis flowers were not mutagenic in an Ames test, and a tea derived from C. officinalis was not genotoxic in Drosophila melanogaster. Clinical testing of cosmetic formulations containing the extract elicited little irritation or sensitization. This review has explored the organoleptic, in-vitro and in-vivo pharmacological activities as well as description, cultivation and active chemical constituents of Calendula officinalisin order to existing information on this plant as well as highlighted its multi activity properties as a medicinal agent.
Creatine analogs having antiviral activity
KADDURAH-DAOUK RIMA, LILLIE JAMES W, WIDLANSKI THEODORE S, BURBAUM JONATHAN J, FORSYTH CRAIG J
Patent: United STATES Patent – United States
Application: US19910812561 on 20 Dec 1991
Publication: 20 Dec 1991
PAT: US5321030
https://europepmc.org/article/PAT/US5321030
Share this article Share with emailShare with twitterShare with linkedinShare with facebook
Abstract
The present invention relates to the use of analogs of creatine, such as cyclocreatine, as antiviral agents. Analogs of creatine can be used as antiviral agents against a variety of viruses, particularly DNA viruses, such as Herpes viruses (e.g., HSV-1, HSV-2, cytomegaloviruses, Varicella-Zoster virus) and adenovirus. The invention further relates to creatine analogs including four classes of creatine analogs selected as candidate antiviral compounds: (1) creatine analogs that can be phosphorylated by creatine kinase but differ in their phosphoryl group transfer potential, (2) bisubstrate inhibitors of creatine kinase comprising covalently linked structural analogs of adenosine triphosphate (ATP) and creatine, (3) creatine analogs which can act as irreversible inhibitors of creatine kinase, and (4) N-phosphorocreatine analogs bearing non-transferable moieties which mimic the N-phosphoryl group.
Antiviral activity of a novel mixture of natural antimicrobials, in vitro, and in a chicken infection model in vivo
Igori Balta, Lavinia Stef, Ioan Pet, Patrick Ward, Todd Callaway, Steven C. icke, Ozan Gundogdu & Nicolae Corcionivoschi
Scientific Reports volume 10, Article number: 16631 (2020) Cite this article
Abstract
The aim of this study was to test in vitro the ability of a mixture of citrus extract, maltodextrin, sodium chloride, lactic acid and citric acid (AuraShield L) to inhibit the virulence of infectious bronchitis, Newcastle disease, avian influenza, porcine reproductive and respiratory syndrome (PRRS) and bovine coronavirus viruses. Secondly, in vivo, we have investigated its efficacy against infectious bronchitis using a broiler infection model. In vitro, these antimicrobials had expressed antiviral activity against all five viruses through all phases of the infection process of the host cells. In vivo, the antimicrobial mixture reduced the virus load in the tracheal and lung tissue and significantly reduced the clinical signs of infection and the mortality rate in the experimental group E2 receiving AuraShield L. All these effects were accompanied by a significant reduction in the levels of pro-inflammatory cytokines and an increase in IgA levels and short chain fatty acids (SCFAs) in both trachea and lungs. Our study demonstrated that mixtures of natural antimicrobials, such AuraShield L, can prevent in vitro viral infection of cell cultures. Secondly, in vivo, the efficiency of vaccination was improved by preventing secondary viral infections through a mechanism involving significant increases in SCFA production and increased IgA levels. As a consequence the clinical signs of secondary infections were significantly reduced resulting in recovered production performance and lower mortality rates in the experimental group E2.
Clin Sci (Lond). 2021 Jan 29;135(2):305-325.
Host cell glutamine metabolism as a potential antiviral target
Sandro Massao Hirabara # 1, Renata Gorjao # 1, Adriana Cristina Levada-Pires 1, Laureane Nunes Masi 1, Elaine Hatanaka 1, Maria Fernanda Cury-Boaventura 1, Eliane Borges da Silva 1, Laiane Cristina Dos Santos-Oliveira 1, Vinicius Leonardo Sousa Diniz 1, Tamires Afonso Duarte Serdan 1, Vivian Araujo Barbosa de Oliveira 1, Diego Ribeiro de Souza 1, Raquel Bragante Gritte 1, Talita Souza-Siqueira 1, Raquel Freitas Zambonatto 1, Tania Cristina Pithon-Curi 1, Roberto Barbosa Bazotte 2 3, Philip Newsholme 4, Rui Curi 1 5
- DOI: 10.1042/CS20201042
Abstract
A virus minimally contains a nucleic acid genome packaged by a protein coat. The genome and capsid together are known as the nucleocapsid, which has an envelope containing a lipid bilayer (mainly phospholipids) originating from host cell membranes. The viral envelope has transmembrane proteins that are usually glycoproteins. The proteins in the envelope bind to host cell receptors, promoting membrane fusion and viral entry into the cell. Virus-infected host cells exhibit marked increases in glutamine utilization and metabolism. Glutamine metabolism generates ATP and precursors for the synthesis of macromolecules to assemble progeny viruses. Some compounds derived from glutamine are used in the synthesis of purines and pyrimidines. These latter compounds are precursors for the synthesis of nucleotides. Inhibitors of glutamine transport and metabolism are potential candidate antiviral drugs. Glutamine is also an essential nutrient for the functions of leukocytes (lymphocyte, macrophage, and neutrophil), including those in virus-infected patients. The increased glutamine requirement for immune cell functions occurs concomitantly with the high glutamine utilization by host cells in virus-infected patients. The development of antiviral drugs that target glutamine metabolism must then be specifically directed at virus-infected host cells to avoid negative effects on immune functions. Therefore, the aim of this review was to describe the landscape of cellular glutamine metabolism to search for potential candidates to inhibit glutamine transport or glutamine metabolism.
bioRxiv. 2020 Oct 4;2020.10.04.325423.
GABA administration prevents severe illness and death following coronavirus infection in mice
Jide Tian, Blake Milddleton, Daniel L Kaufman
Abstract
There is an urgent need for new treatments to prevent and ameliorate severe illness and death induced by SARS-CoV-2 infection in COVID-19 patients. The coronavirus mouse hepatitis virus (MHV)-1 causes pneumonitis in mice which shares many pathological characteristics with human SARS-CoV infection. Previous studies have shown that the amino acid gamma-aminobutyric acid (GABA) has anti-inflammatory effects. We tested whether oral treatment with GABA could modulate the MHV-1 induced pneumonitis in susceptible A/J mice. As expected, MHV-1-inoculated control mice became severely ill (as measured by weight loss, clinical score, and the ratio of lung weight to body weight) and >60% of them succumbed to the infection. In contrast, mice that received GABA immediately after MHV-1 inoculation became only mildly ill and all of them recovered. When GABA treatment was initiated after the appearance of illness (3 days post-MHV-1 infection), we again observed that GABA treatment significantly reduced the severity of illness and greatly increased the frequency of recovery. Therefore, the engagement of GABA receptors (GABA-Rs) prevented the MHV-1 infection-induced severe pneumonitis and death in mice. Given that GABA-R agonists, like GABA and homotaurine, are safe for human consumption, stable, inexpensive, and available worldwide, they are promising candidates to help prevent severe illness stemming from SARS-CoV-2 infection and other coronavirus strains.
GABA administration limits viral replication and pneumonitis in a mouse model of COVID-19
Jide Tian, Blake Middleton, Daniel L. Kaufman
doi: https://doi.org/10.1101/2021.02.09.430446
This article is a preprint and has not been certified by peer review [what does this mean?].
Abstract
Despite the availability of vaccines for COVID-19, serious illness and death induced by coronavirus infection will remain a global health burden because of vaccination hesitancy, possible virus mutations, and the appearance of novel coronaviruses. Accordingly, there is a need for new approaches to limit severe illness stemming from coronavirus infections. Cells of the immune system and lung epithelia express receptors for GABA (GABA-Rs), a widely used neurotransmitter within the CNS. GABA-R agonists have anti-inflammatory effects and can limit acute lung injury. We previously showed that GABA treatment effectively reduced disease severity and death rates in mice following infection with a coronavirus (MHV-1) which provides a potentially lethal model of COVID-19. Here, we report that GABA treatment also reduced viral load in the lungs, suggesting that GABA-Rs may provide a new druggable target to limit pulmonary coronavirus replication. Histopathological analysis revealed that GABA treatment reduced lung inflammatory infiltrates and damages. Since GABA is safe for human consumption, inexpensive, and available worldwide, it is a promising candidate to help treat COVID-19.
Dermatol Ther. 2021 Jan;34(1):e14454.
COVID-19: Topical agents and therapeutic prevention of nasal viral acquisition
Shaden Abdelhadi 1, Zbigniew Ruszczak 2, Robert A Schwartz 3
- DOI: 10.1111/dth.14454
Abstract
Since the spread of SARS-CoV-2 became a pandemic, the number of cases has been continuously growing worldwide. Numerous recommendations and suggestions have been published to prevent the acquisition and spread of the SARS-CoV-2, especially to protect health workers and front-line caregivers. SARS-CoV-2 is transmitted by aerosol, rendering air defense with suitable ventilation and adequate mask use pivotal. Recently, locally applied antiseptic, antiviral, or structure competitive receptor blockers were suggested to attack the virus at its main point of invasion, the nasal mucosa and nasopharynx. We discuss the most plausible and safe ideas to reduce viral load at the point of entry, and subsequently the spread of SARS-CoV-2 to the lower respiratory tract, lungs, and other organs. In addition, we analyze the value and recommend clinical trials employing topical trichloroacetic acid (TCA), a substance well known from dermatologic and cosmetic procedures. It has been proven to successfully block the nasal entry for airborne allergens, preventing the development of allergic rhinitis and asthma, and to be curative for early stages of viral infections entering through the oral mucosa. For SARS-CoV-2, TCA in a single, short-time application is expected to remodel the nasal and nasopharyngeal epithelia, eliminating both the receptors and cells responsible for viral entry and subsequent viral spread to the lower respiratory tract. Moreover, this may have therapeutic benefits for those recently infected by reducing local viral replication. Such procedures are cheap, safe, and can be conducted in almost every setting, especially in regions with inadequate financial and logistic resources.
Biomed Pharmacother. 2020 Nov; 131: 110694.
Published online 2020 Aug 27. doi: 10.1016/j.biopha.2020.110694
Can a metabolism-targeted therapeutic intervention successfully subjugate SARS-COV-2? A scientific rational
Kamran Mansouri,a,1 Mohsen Rastegari-Pouyani,b,1 Maryam Ghanbri-Movahed,a,c Mehrnoush Safarzadeh,b Sara Kiani,a and Zahra Ghanbari-Movaheda,*
Keywords: SARS-CoV-2, Cellular metabolism, Glycolysis, PPP, TCA cycle
Abstract
As a process entailing a high turnover of the host cell molecules, viral replication is required for a successful viral infection and requests virus capacity to acquire the macromolecules required for its propagation. To this end, viruses have adopted several strategies to harness cellular metabolism in accordance with their specific demands. Most viruses upregulate specific cellular anabolic pathways and are largely dependent on such alterations. RNA viruses, for example, upregulate both glycolysisand glycogenolysis providing TCA cycle intermediates essential for anabolic lipogenesis. Also, these infections usually induce the PPP, leading to increased nucleotide levels supporting viral replication. SARS-CoV-2 (the cause of COVID-19)that has so far spread from China throughout the world is also an RNA virus. Owing to the more metabolic plasticity of uninfected cells, a promising approach for specific antiviral therapy, which has drawn a lot of attention in the recent years,
would be the targeting of metabolic changes induced by viruses. In the current review, we first summarize some of virus-induced metabolic adaptations and then based on these information as well as SARS-CoV-2 pathogenesis, propose a potential therapeutic modality for this calamitous world-spreading virus with the hope of employing this strategy for near-future clinical application.
As a process entailing a high turnover of the host cell molecules, viral replication is required for a successful viral infection and requests virus capacity to acquire the macromolecules required for its propagation. To this end, viruses have adopted several strategies to harness cellular metabolism in accordance with their specific demands. Most viruses upregulate specific cellular anabolic pathways and are largely dependent on such alterations. RNA viruses, for example, upregulate both glycolysisand glycogenolysis providing TCA cycle intermediates essential for anabolic lipogenesis. Also, these infections usually induce the PPP, leading to increased nucleotide levels supporting viral replication. SARS-CoV-2 (the cause of COVID-19)that has so far spread from China throughout the world is also an RNA virus. Owing to the more metabolic plasticity of uninfected cells, a promising approach for specific antiviral therapy, which has drawn a lot of attention in the recent years,
would be the targeting of metabolic changes induced by viruses. In the current review, we first summarize some of virus-induced metabolic adaptations and then based on these information as well as SARS-CoV-2 pathogenesis, propose a potential therapeutic modality for this calamitous world-spreading virus with the hope of employing this strategy for near-future clinical application.
J Biomol Struct Dyn. 2021 Mar 24;1-12.
A computational study on active constituents of Habb-ul-aas and Tabasheer as inhibitors of SARS-CoV-2 main protease
Shariq Shamsi 1, Hina Anjum 1, Mohd Shahbaaz 2 3, Mohd Shahnawaz Khan 4, Farid S Ataya 4, Alya Alamri 4, Fahad A Alhumaydhi 5, Fohad Mabood Husain 6, Md Tabish Rehman 7, Taj Mohammad 8, Asimul Islam 8, Farah Anjum 9, Anas Shamsi 8 10
Abstract
A respiratory pandemic known as coronavirus disease-19 (COVID-19) has created havoc since it emerged from Wuhan, China. COVID-19 is caused by a newly emerged SARS coronavirus (SARS-CoV) with increased pathogenicity named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Due to the lack of understanding of the mechanism of pathogenesis, an effective therapeutic option is unavailable. Epidemics described in Unani ancient literature include nazla-e-wabai and humma-e-wabai, and most of the symptoms of COVID-19 resemble nazla-e-wabai. Hence, in light of Unani literature, the treatment of COVID-19 can be managed with the composites prescribed in Unani medicine for nazla-e-wabai. In this study, a structure-based drug design approach was carried out to check the effectiveness of the pharmacologically active constituents of the Unani composites prescribed to treat nazla-e-wabai against SARS-CoV-2. We performed molecular docking of the active constituents of these composites against the main protease (Mpro), a potential drug target in SARS-CoV-2. Using detailed molecular docking analysis, Habb-ul-aas and Tabasheer were identified as potential inhibitors of SARS-CoV-2 Mpro. The active constituents of both these composites bind to the substrate-binding pocket of SARS-CoV-2 Mpro, forming interactions with key residues of the binding pocket. Molecular dynamics (MD) simulation suggested the binding of active constituents of Habb-ul-aas with SARS-CoV-2 Mpro with a strong affinity as compared to the constituents of Tabasheer. Thus, this study sheds light on the use of these Unani composites in COVID-19 therapeutics.Communicated by Ramaswamy H. Sarma.
Pharmacol Res Commun. 1986 Nov;18(11):1063-73.
Antiviral activities of gossypol and its derivatives against herpes simplex virus type II
R J Radloff, L M Deck, R E Royer, D L Vander Jagt
Abstract
Gossypol, a disequiterpene obtained from cottonseed oil, and a series of peri-acylated gossylic nitriles were compared for their antiviral activities against HSV-II and for their toxicities to the host Vero cells. All of the peri-acylated gossylic nitriles exhibited lower cytotoxicities to the host cell than did the parent compound gossypol. Both gossypol and the series of derivatives exhibited antiviral activities against HSV-II when the virus was treated with drug at concentrations as low as 5 X 10(-7) M. Two of the derivatives, gossylic nitrile-1,1′-diacetate and gossylic nitrile-1,1′-divalerate, were capable of inhibiting viral multiplication in Vero cells that were infected with virus before administration of the drug. The results of this study indicate that modification of the aldehyde functional groups on gossypol lowers the toxicity of this drug but does not abolish its antiviral properties. Derivatives of gossypol may be useful antiviral agents.
Evidence for treatment with estradiol for women with SARS-CoV-2 infection
- Ute Seeland, Flaminia Coluzzi, Maurizio Simmaco, Cameron Mura, Philip E. Bourne, Max Heiland, Robert Preissner & Saskia Preissner
BMC Medicine volume 18, Article number: 369 (2020) Cite this article
Abstract
Background
Given that an individual’s age and gender are strongly predictive of coronavirus disease 2019 (COVID-19) outcomes, do such factors imply anything about preferable therapeutic options?
Methods
An analysis of electronic health records for a large (68,466-case), international COVID-19 cohort, in 5-year age strata, revealed age-dependent sex differences. In particular, we surveyed the effects of systemic hormone administration in women. The primary outcome for estradiol therapy was death. Odds ratios (ORs) and Kaplan-Meier survival curves were analyzed for 37,086 COVID-19 women in two age groups: pre- (15–49 years) and peri-/post-menopausal (> 50 years).
Results
The incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is higher in women than men (by about + 15%) and, in contrast, the fatality rate is higher in men (about + 50%). Interestingly, the relationships between these quantities are linked to age: pre-adolescent girls and boys had the same risk of infection and fatality rate, while adult premenopausal women had a significantly higher risk of infection than men in the same 5-year age stratum (about 16,000 vs. 12,000 cases). This ratio changed again in peri- and postmenopausal women, with infection susceptibility converging with men. While fatality rates increased continuously with age for both sexes, at 50 years, there was a steeper increase for men. Thus far, these types of intricacies have been largely neglected. Because the hormone 17ß-estradiol influences expression of the human angiotensin-converting enzyme 2 (ACE2) protein, which plays a role in SARS-CoV-2 cellular entry, propensity score matching was performed for the women’s sub-cohort, comparing users vs. non-users of estradiol. This retrospective study of hormone therapy in female COVID-19 patients shows that the fatality risk for women > 50 years receiving estradiol therapy (user group) is reduced by more than 50%; the OR was 0.33, 95% CI [0.18, 0.62] and the hazard ratio (HR) was 0.29, 95% CI [0.11,0.76]. For younger, pre-menopausal women (15–49 years), the risk of COVID-19 fatality is the same irrespective of estradiol treatment, probably because of higher endogenous estradiol levels.
Conclusions
As of this writing, still no effective drug treatment is available for COVID-19; since estradiol shows such a strong improvement regarding fatality in COVID-19, we suggest prospective studies on the potentially more broadly protective roles of this naturally occurring hormone.
Sci Rep. 2020 Oct 1;10(1):16200.
Antiviral activity of digoxin and ouabain against SARS-CoV-2 infection and its implication for COVID-19
Junhyung Cho 1, Young Jae Lee 1, Je Hyoung Kim 1, Sang Il Kim 2, Sung Soon Kim 3, Byeong-Sun Choi 4, Jang-Hoon Choi 5
Free PMC article
Abstract
The current coronavirus (COVID-19) pandemic is exacerbated by the absence of effective therapeutic agents. Notably, patients with COVID-19 and comorbidities such as hypertension and cardiac diseases have a higher mortality rate. An efficient strategy in response to this issue is repurposing drugs with antiviral activity for therapeutic effect. Digoxin (DIG) and ouabain (OUA) are FDA drugs for heart diseases that have antiviral activity against several coronaviruses. Thus, we aimed to assess antiviral activity of DIG and OUA against SARS-CoV-2 infection. The half-maximal inhibitory concentrations (IC50) of DIG and OUA were determined at a nanomolar concentration. Progeny virus titers of single-dose treatment of DIG, OUA and remdesivir were approximately 103-, 104– and 103-fold lower (> 99% inhibition), respectively, than that of non-treated control or chloroquine at 48 h post-infection (hpi). Furthermore, therapeutic treatment with DIG and OUA inhibited over 99% of SARS-CoV-2 replication, leading to viral inhibition at the post entry stage of the viral life cycle. Collectively, these results suggest that DIG and OUA may be an alternative treatment for COVID-19, with potential additional therapeutic effects for patients with cardiovascular disease.
An Acad Bras Cienc. 2020 Oct 28;92(4):e20201080.
Cardiac glycosides and COVID-19: would it be a promising therapeutic approach?
Eftychios Siniorakis 1, Spyridon Arvanitakis 1, Maximilianos Elkouris 2
Abstract
Cardiac glycosides have been found to have an anti-viral effect. This was noted in the past during various epidemics including MERS and SARS. It is due to their inhibitory effect on the Na, K-ATPase membrane pump. Furthermore, they exhibit anti-inflammatory properties. These preclinical observations may prove useful in further clinical utility of these well-known compounds in the current COVID-19 pandemic.
Biomolecules. 2020 Jun; 10(6): 832.
Published online 2020 May 29. doi: 10.3390/biom10060832
Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back
Hana Vylíčilová,1,† Magdaléna Bryksová,1,† Vlasta Matušková,1 Karel Doležal,1,2 Lucie Plíhalová,1,2,* and Miroslav Strnad2
Abstract
Cytokinins and their sugar or non-sugar conjugates are very active growth-promoting factors in plants, although they occur at very low concentrations. These compounds have been identified in numerous plant species. This review predominantly focuses on 9-substituted adenine-based cytokinin conjugates, both artificial and endogenous, sugar and non-sugar, and their roles in plants. Acquired information about their biological activities, interconversions, and metabolism improves understanding of their mechanisms of action and functions in planta. Although a number of 9-substituted cytokinins occur endogenously, many have also been prepared in laboratories to facilitate the clarification of their physiological roles and the determination of their biological properties. Here, we chart advances in knowledge of 9-substituted cytokinin conjugates from their discovery to current understanding and reciprocal interactions between biological properties and associated structural motifs. Current organic chemistry enables preparation of derivatives with better biological properties, such as improved anti-senescence, strong cell division stimulation, shoot forming, or more persistent stress tolerance compared to endogenous or canonical cytokinins. Many artificial cytokinin conjugates stimulate higher mass production than naturally occurring cytokinins, improve rooting, or simply have high stability or bioavailability. Thus, knowledge of the biosynthesis, metabolism, and activity of 9-substituted cytokinins in various plant species extends the scope for exploiting both natural and artificially prepared cytokinins in plant biotechnology, tissue culture, and agriculture.
Cytokinins and their sugar or non-sugar conjugates are very active growth-promoting factors in plants, although they occur at very low concentrations. These compounds have been identified in numerous plant species. This review predominantly focuses on 9-substituted adenine-based cytokinin conjugates, both artificial and endogenous, sugar and non-sugar, and their roles in plants. Acquired information about their biological activities, interconversions, and metabolism improves understanding of their mechanisms of action and functions in planta. Although a number of 9-substituted cytokinins occur endogenously, many have also been prepared in laboratories to facilitate the clarification of their physiological roles and the determination of their biological properties. Here, we chart advances in knowledge of 9-substituted cytokinin conjugates from their discovery to current understanding and reciprocal interactions between biological properties and associated structural motifs. Current organic chemistry enables preparation of derivatives with better biological properties, such as improved anti-senescence, strong cell division stimulation, shoot forming, or more persistent stress tolerance compared to endogenous or canonical cytokinins. Many artificial cytokinin conjugates stimulate higher mass production than naturally occurring cytokinins, improve rooting, or simply have high stability or bioavailability. Thus, knowledge of the biosynthesis, metabolism, and activity of 9-substituted cytokinins in various plant species extends the scope for exploiting both natural and artificially prepared cytokinins in plant biotechnology, tissue culture, and agriculture.
Eur J Med Chem. 2015 Jan 7; 89: 421–441.
Published online 2014 Oct 23. doi: 10.1016/j.ejmech.2014.10.065
A review on recent developments of indole-containing antiviral agents
Ming-Zhi Zhang,a Qiong Chen,a,∗ and Guang-Fu Yanga,b,∗
Abstract
Indole represents one of the most important privileged scaffolds in drug discovery. Indole derivatives have the unique property of mimicking the structure of peptides and to bind reversibly to enzymes, which provide tremendous opportunities to discover novel drugs with different modes of action. There are seven indole-containing commercial drugs in the Top-200 Best Selling Drugs by US Retail Sales in 2012. There are also an amazing number of approved indole-containing drugs in the market as well as compounds currently going through different clinical phases or registration statuses. This review focused on the recent development of indole derivatives as antiviral agents with the following objectives: 1) To present one of the most comprehensive listings of indole antiviral agents, drugs on market or compounds in clinical trials; 2) To focus on recent developments of indole compounds (including natural products) and their antiviral activities, summarize the structure property, hoping to inspire new and even more creative approaches; 3) To offer perspectives on how indole scaffolds as a privileged structure might be exploited in the future.Indole represents one of the most important privileged scaffolds in drug discovery. Indole derivatives have the unique property of mimicking the structure of peptides and to bind reversibly to enzymes, which provide tremendous opportunities to discover novel drugs with different modes of action. There are seven indole-containing commercial drugs in the Top-200 Best Selling Drugs by US Retail Sales in 2012. There are also an amazing number of approved indole-containing drugs in the market as well as compounds currently going through different clinical phases or registration statuses. This review focused on the recent development of indole derivatives as antiviral agents with the following objectives: 1) To present one of the most comprehensive listings of indole antiviral agents, drugs on market or compounds in clinical trials; 2) To focus on recent developments of indole compounds (including natural products) and their antiviral activities, summarize the structure property, hoping to inspire new and even more creative approaches; 3) To offer perspectives on how indole scaffolds as a privileged structure might be exploited in the future.
Sci Data. 2021 Feb 26;8(1):70.
A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection
Bernhard Ellinger # 1, Denisa Bojkova # 2, Andrea Zaliani 3, Jindrich Cinatl 2, Carsten Claussen 3 4, Sandra Westhaus 2, Oliver Keminer 3, Jeanette Reinshagen 3, Maria Kuzikov 3, Markus Wolf 3, Gerd Geisslinger 5 6 4, Philip Gribbon 3 4, Sandra Ciesek 2 6 7
Abstract
SARS-CoV-2 is a novel coronavirus responsible for the COVID-19 pandemic, in which acute respiratory infections are associated with high socio-economic burden. We applied high-content screening to a well-defined collection of 5632 compounds including 3488 that have undergone previous clinical investigations across 600 indications. The compounds were screened by microscopy for their ability to inhibit SARS-CoV-2 cytopathicity in the human epithelial colorectal adenocarcinoma cell line, Caco-2. The primary screen identified 258 hits that inhibited cytopathicity by more than 75%, most of which were not previously known to be active against SARS-CoV-2 in vitro. These compounds were tested in an eight-point dose response screen using the same image-based cytopathicity readout. For the 67 most active molecules, cytotoxicity data were generated to confirm activity against SARS-CoV-2. We verified the ability of known inhibitors camostat, nafamostat, lopinavir, mefloquine, papaverine and cetylpyridinium to reduce the cytopathic effects of SARS-CoV-2, providing confidence in the validity of the assay. The high-content screening data are suitable for reanalysis across numerous drug classes and indications and may yield additional insights into SARS-CoV-2 mechanisms and potential therapeutic strategies.
Journal of Virology. Vaccines and Antiviral Agents
Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses
Megha Aggarwal, George P. Leser, Robert A. Lamb
DOI: 10.1128/JVI.01888-19
ABSTRACT
Influenza viruses are highly infectious and are the leading cause of human respiratory diseases and may trigger severe epidemics and occasional pandemics. Although antiviral drugs against influenza viruses have been developed, there is an urgent need to design new strategies to develop influenza virus inhibitors due to the increasing resistance of viruses toward currently available drugs. In this study, we examined the antiviral activity of natural compounds against the following influenza virus strains: A/WSN/33 (H1N1), A/Udorn/72 (H3N2), and B/Lee/40. Papaverine (a nonnarcotic alkaloid that has been used for the treatment of heart disease, impotency, and psychosis) was found to be an effective inhibitor of multiple strains of influenza virus. Kinetic studies demonstrated that papaverine inhibited influenza virus infection at a late stage in the virus life cycle. An alteration in influenza virus morphology and viral ribonucleoprotein (vRNP) localization was observed as an effect of papaverine treatment. Papaverine is a well-known phosphodiesterase inhibitor and also modifies the mitogen-activated protein kinase (MAPK) pathway by downregulating the phosphorylation of MEK and extracellular signal-regulated kinase (ERK). Thus, the modulation of host cell signaling pathways by papaverine may be associated with the nuclear retention of vRNPs and the reduction of influenza virus titers. Interestingly, papaverine also inhibited paramyxoviruses parainfluenza virus 5 (PIV5), human parainfluenza virus 3 (HPIV3), and respiratory syncytial virus (RSV) infections. We propose that papaverine can be a potential candidate to be used as an antiviral agent against a broad range of influenza viruses and paramyxoviruses.
IMPORTANCE Influenza viruses are important human pathogens that are the causative agents of epidemics and pandemics. Despite the availability of an annual vaccine, a large number of cases occur every year globally. Here, we report that papaverine, a vasodilator, shows inhibitory action against various strains of influenza virus as well as the paramyxoviruses PIV5, HPIV3, and RSV. A significant effect of papaverine on the influenza virus morphology was observed. Papaverine treatment of influenza-virus-infected cells resulted in the inhibition of virus at a later time in the virus life cycle through the suppression of nuclear export of vRNP and also interfered with the host cellular cAMP and MEK/ERK cascade pathways. This study explores the use of papaverine as an effective inhibitor of both influenza viruses as well as paramyxoviruses.
Human coronaviruses and therapeutic drug discovery
Lan-Gui Song, Qing-Xing Xie, Hui-Lin Lao & Zhi-Yue Lv
Infectious Diseases of Poverty volume 10, Article number: 28 (2021) Cite this article
Abstract
Background
Coronaviruses (CoVs) are distributed worldwide and have various susceptible hosts; CoVs infecting humans are called human coronaviruses (HCoVs). Although HCoV-specific drugs are still lacking, many potent targets for drug discovery are being explored, and many vigorously designed clinical trials are being carried out in an orderly manner. The aim of this review was to gain a comprehensive understanding of the current status of drug development against HCoVs, particularly severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Main text
A scoping review was conducted by electronically searching research studies, reviews, and clinical trials in PubMed and the CNKI. Studies on HCoVs and therapeutic drug discovery published between January 2000 and October 2020 and in English or Chinese were included, and the information was summarized. Of the 3248 studies identified, 159 publication were finally included. Advances in drug development against HCoV, especially SARS-CoV-2, are summarized under three categories: antiviral drugs aimed at inhibiting the HCoV proliferation process, drugs acting on the host’s immune system, and drugs derived from plants with potent activity. Furthermore, clinical trials of drugs targeting SARS-CoV-2 are summarized.
Conclusions
During the spread of COVID-19 outbreak, great efforts have been made in therapeutic drug discovery against the virus, although the pharmacological effects and adverse reactions of some drugs under study are still unclear. However, well-designed high-quality studies are needed to further study the effectiveness and safety of these potential drugs so as to provide valid recommendations for better control of the COVID-19 pandemic.
Quote: “Aster pentapeptide A, ligustrazine, salvianolic acid B, etc., have potential inhibitory effects on SARS-COV-2 Mpro”
Pharm Biol. 2013 Sep;51(9):1137-43.
Antiviral effects of the constituents derived from Chinese herb medicines on infectious bursal disease virus
Yaogui Sun 1, Meiqin Song, Li Niu, Xiyun Bai, Na Sun, Xin Zhao, Junbing Jiang, Junping He, Hongquan Li
Affiliations expand
Abstract
Context: The prevalence of infectious bursal disease has brought about enormous financial losses to the world poultry industry. Chinese herb medicines can provide valuable materials for discovery and development of new drugs.
Objective: To screen constituents derived from Chinese herb medicines for their antiviral activity against infectious bursal disease virus (IBDV) in vitro.
Materials and methods: Twenty constituents derived from Chinese herb medicines and B87 strain of IBDV were used. The 50% cytotoxic concentration (CC₅₀) and 50% effective concentration (EC₅₀) were determined by visualization of cytopathologic effect (CPE) and 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) test on chicken embryo fibroblast. Selectivity index (SI) and inhibition ratio (%I) were calculated from the data obtained from the MTT test.
Results: Antiviral assays showed dipotassium glycyrrhizinate and ligustrazine hydrochloride among the 20 constituents tested exhibited significant inhibitory activity against IBDV in a dose-dependent manner. EC₅₀ of dipotassium glycyrrhizinate and ligustrazine hydrochloride were 663.2 ± 268.4 and 92.52 ± 21.13 µg/mL, and SI were >4.52 and >21.62, respectively. The time-of-addition and virucidal assay indicated that anti-IBDV activity of the two constituents could be due to their inhibiting virus replication and/or inactivating virus directly. The inhibition of virus attachment was not observed in the adsorption inhibition assay. Dipotassium glycyrrhizinate and ligustrazine hydrochloride exhibited more than 70% and 80% inhibition of IBDV, respectively, at the maximum safe concentration.
Discussion and conclusion: We believe that dipotassium glycyrrhizinate and ligustrazine hydrochloride can be used to develop a new anti-IBDV compound, and it is worth applying the constituents in clinical practice.
Front. Nutr., 29 October 2020 | https://doi.org/10.3389/fnut.2020.562051
Potential Role of Vitamin B6 in Ameliorating the Severity of COVID-19 and Its Complications
Thanutchaporn Kumrungsee1*, Peipei Zhang2, Maesaya Chartkul3,4,5, Noriyuki Yanaka1 and Norihisa Kato1*
Introduction
The word is currently experiencing a coronavirus disease-19 (COVID-19) pandemic caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). Coronaviruses and influenza are among the viruses that can cause lethal lung injuries and death from acute respiratory distress syndrome worldwide (1). Viral infections evoke a “cytokine storm,” leading to lung capillary endothelial cell inflammation, neutrophil infiltration, and increased oxidative stress (1, 2). Furthermore, cardiovascular and diabetic complications are emerging in COVID-19 patients (3–9). Currently, there is no registered treatment or vaccine for COVID-19, and an alternative solution to protect against COVID-19 is urgently needed.
Vitamin B6 is a water-soluble vitamin found in various foods such as fish, whole grains, and banana (10). There are six isoforms of B6 vitamers (10). Among these, pyridoxal 5′-phosphate (PLP) is the most active form that acts as a coenzyme in various enzymatic reactions (10). There is growing evidence that vitamin B6 exerts a protective effect against chronic diseases such as cardiovascular diseases (CVD) and diabetes by suppressing inflammation, inflammasomes, oxidative stress, and carbonyl stress (11). Additionally, vitamin B6 deficiency is associated with lower immune function and higher susceptibility to viral infection (12, 13). In view of these information, we postulated potential role of vitamin B6 in ameliorating the severity of COVID-19 and its complications (Figure 1). In this article, we review precedent research to test this hypothesis.
Maturitas. 2021 Feb; 144: 108–111.
Published online 2020 Aug 15. doi: 10.1016/j.maturitas.2020.08.007
Be well: A potential role for vitamin B in COVID-19
Hira Shakoor,a Jack Feehan,b,c Kathleen Mikkelsen,b Ayesha S. Al Dhaheri,a Habiba I. Ali,a Carine Platat,a Leila Cheikh Ismail,d,e Lily Stojanovska,a,b and Vasso Apostolopoulosb,*
Author information Article notes Copyright and License information Disclaimer
This article has been cited by other articles in PMC.
Coronavirus disease (COVID-19) is caused by the SARS-CoV-2 virus. In January 2020, the World Health Organization declared COVID-19 as a Public Health Emergency of International Concern and in March 2020, COVID-19 was characterized as a global pandemic that is responsible for infecting over 20 million and more than 700,000 deaths. COVID-19 symptoms are fever, cough, fatigue, headache, diarrhea, arthromyalgias, serious interstitial pneumonia that can lead to acute respiratory distress syndrome, sepsis-induced coagulopathy and multi-organ dysfunction [1]. In addition, the severe progression of COVID-19 results in cytokine storm with excessive production of pro-inflammatory cytokines [2]. Previously, outbreaks of similar viruses which belong to the β-coronavirus family occurred in 2002–2004 and 2012–2014, as severe acute respiratory syndrome (SARS) and as the Middle East respiratory syndrome (MERS), respectively [3,4].
Currently, there is no approved drug treatment or vaccine against the SARS-CoV-2 virus. Until these become available, one must include adequate and balanced nutrition for proper body functioning and boosting of the immune system. Micronutrients, vitamin C and vitamin D have gained much attention during the pandemic because of their anti-inflammatory and immune-supporting properties. Low levels of vitamins D and C result in coagulopathy and suppress the immune system, causing lymphocytopenia. Evidence has shown that the mortality rate is higher in COVID-19 patients with low vitamin D concentrations. Further, vitamin C supplementation increases the oxygenation index in COVID-19 infected patients [5]. Similarly, vitamin B deficiency can significantly impair cell and immune system function, and lead to inflammation due to hyperhomocysteinemia.
There is a need to highlight the importance of vitamin B because it plays a pivotal role in cell functioning, energy metabolism, and proper immune function [6]. Vitamin B assists in proper activation of both the innate and adaptive immune responses, reduces pro-inflammatory cytokine levels, improves respiratory function, maintains endothelial integrity, prevents hypercoagulability and can reduce the length of stay in hospital [7,8]. Therefore, vitamin B status should be assessed in COVID-19 patients and vitamin B could be used as a non-pharmaceutical adjunct to current treatments.
Antimicrob Agents Chemother. 1994 Oct;38(10):2483-7.
Inhibition of gp120-CD4 interaction and human immunodeficiency virus type 1 infection in vitro by pyridoxal 5′-phosphate
L Guo 1, N K Heinzinger, M Stevenson, L M Schopfer, J M Salhany
Abstract
Pyridoxal 5′-phosphate and related compounds were tested for their ability to inhibit gp120-CD4 interaction and human immunodeficiency virus infection in vitro. The results show that pyridoxal 5′-phosphate is a unique CD4 antagonist whose antiviral potency derives from the presence of both lysine-reactive and anionic substituents.
J Biomol Struct Dyn. 2020 : 1–8.
Published online 2020 Oct 23. doi: 10.1080/07391102.2020.1835732
Caffeine and caffeine-containing pharmaceuticals as promising inhibitors for 3-chymotrypsin-like protease of SARS-CoV-2
Abstract
In December 2019, a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to the outbreak of a pulmonary disease called COVID-19, which killed thousands of people worldwide. Therefore, the necessity to find out the potential therapeutic pharmaceuticals is imperious. This study investigates the inhibitory effect of SARS-CoV-2 3-chymotrypsin-like protease (3CLpro) using caffeine and caffeine-containing pharmaceuticals (3CPs) based on molecular dynamics simulations and free energy calculations by means of molecular mechanics-Poisson–Boltzmann surface area (MMPBSA) and molecular mechanics-generalized-Born surface area (MMGBSA). Of these 3CPs, seven drugs approved by the US-Food and Drug Administration have shown a good binding affinity to the catalytic residues of 3CLpro of His41 and Cys145: caffeine, theophylline, dyphylline, pentoxifylline, linagliptin, bromotheophylline and istradefylline. Their binding affinity score ranged from –4.9 to –8.6 kcal/mol. The molecular dynamic simulation in an aqueous solution of docked complexes demonstrated that the 3CPs conformations bound to the active sites of 3CLpro during 200 ns molecular dynamics simulations. The free energy of binding also confirms the stability of the 3CPs–3CLpro complexes. To our knowledge, this in silico study shows for the first time very inexpensive drugs available in large quantities that can be potential inhibitors against 3CLpro. In particular, the repurposing of linagliptin, and caffeine are recommended for COVID-19 treatment after in vitro, in vivo and clinical trial validation.In December 2019, a new coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to the outbreak of a pulmonary disease called COVID-19, which killed thousands of people worldwide. Therefore, the necessity to find out the potential therapeutic pharmaceuticals is imperious. This study investigates the inhibitory effect of SARS-CoV-2 3-chymotrypsin-like protease (3CLpro) using caffeine and caffeine-containing pharmaceuticals (3CPs) based on molecular dynamics simulations and free energy calculations by means of molecular mechanics-Poisson–Boltzmann surface area (MMPBSA) and molecular mechanics-generalized-Born surface area (MMGBSA). Of these 3CPs, seven drugs approved by the US-Food and Drug Administration have shown a good binding affinity to the catalytic residues of 3CLpro of His41 and Cys145: caffeine, theophylline, dyphylline, pentoxifylline, linagliptin, bromotheophylline and istradefylline. Their binding affinity score ranged from –4.9 to –8.6 kcal/mol. The molecular dynamic simulation in an aqueous solution of docked complexes demonstrated that the 3CPs conformations bound to the active sites of 3CLpro during 200 ns molecular dynamics simulations. The free energy of binding also confirms the stability of the 3CPs–3CLpro complexes. To our knowledge, this in silico study shows for the first time very inexpensive drugs available in large quantities that can be potential inhibitors against 3CLpro. In particular, the repurposing of linagliptin, and caffeine are recommended for COVID-19 treatment after in vitro, in vivo and clinical trial validation.
Food Chem Toxicol. 2008 Jun;46(6):1919-24.
Antiviral activities of coffee extracts in vitro
Hirotoshi Utsunomiya 1, Masao Ichinose, Misao Uozaki, Kazuko Tsujimoto, Hisashi Yamasaki, A Hajime Koyama
Abstract
Both hot water extracts of coffee grinds and instant coffee solutions inhibited the multiplication of herpes simplex virus type 1, a representative enveloped DNA virus, when they were added to the culture medium of the virus-infected cells at a dose of one fifth the concentration suitable for drinking. The antiherpetic activity was independent of the suppliers (companies) of the coffee grinds and of the locations where the coffee beans were produced. Further characterization revealed that there are two different mechanisms, by which the coffee extracts exert inhibitory activities on the virus infection; (1) a direct inactivation of the infectivity of virus particle (i.e., a virucidal activity) and (2) the inhibition of progeny infectious virus formation at the late stage of viral multiplication in the infected cells. Caffeine, but not quinic acid and chlorogenic acid, inhibited the virus multiplication to some extent, but none of them showed the virucidal activity, suggesting that other component(s) in the coffee extracts must play a role in the observed antiviral activity. In addition, the coffee extracts inhibited the multiplication of poliovirus, a non-enveloped RNA virus, but showed no virucidal effect on this virus.
Mol Med Rep. Mar-Apr 2008;1(2):251-5.
Effect of caffeine on the multiplication of DNA and RNA viruses
Masaki Murayama 1, Kazuko Tsujimoto, Misao Uozaki, Yukiko Katsuyama, Hisashi Yamasaki, Hirotoshi Utsunomiya, A Hajime Koyama
https://pubmed.ncbi.nlm.nih.gov/21479405/
Abstract
The present study examined the effect of caffeine on RNA and DNA viruses, revealing that it inhibits the multiplication of both. In the presence of caffeine, the progeny virus yield of both herpes simplex virus type 1 (HSV-1) and poliovirus decreased with increasing concentrations of the reagent, although HSV-1 was much more sensitive than poliovirus. The influenza virus was not affected by caffeine at the same concentrations. None of the viruses were directly inactivated by caffeine at the tested concentrations. Characterization of the mode of action of caffeine against HSV-1 infection revealed that the addition of the reagent at 10 h post-infection significantly affected the formation of progeny virus, indicating that caffeine can inhibit the multiplication of HSV-1 during the step(s) following the completion of viral DNA replication and the formation of nucleocapsids. In addition, the reagent selectively enhanced the cytopathic effects and cell death of the infected cells over uninfected cells, suggesting that the antiviral action of caffeine against HSV-1 is, at least in part, the result of accelerated degeneration of the infected cells.
Virology Volume 335, Issue 2, 10 May 2005, Pages 177-184
Inhibition of HIV-1 replication by caffeine and caffeine-related methylxanthines
GiuseppeNunnariEliasArgyrisJianhuaFangKetti E.MehlmanRoger J.PomerantzRenéDaniel
https://doi.org/10.1016/j.virol.2005.02.015Get rights and content
Abstract
Human immunodeficiency virus type I (HIV-1) DNA integration is an essential step of viral replication. We have suggested recently that this stage of HIV-1 life-cycle triggers a cellular DNA damage response and requires cellular DNA repair proteins for its completion. These include DNA-PK (DNA-dependent protein kinase), ATR (ataxia telangiectasia and Rad3-related), and, at least in some circumstances, ATM (ataxia telangiectasia mutated). Host cell proteins may constitute an attractive target for anti-HIV-1 therapeutics, since development of drug resistance against compounds targeting these cellular cofactor proteins is unlikely. In this study, we show that an inhibitor of ATR and ATM kinases, caffeine, can suppress replication of infectious HIV-1 strains, and provide evidence that caffeine exerts its inhibitory effect at the integration step of the HIV-1 life-cycle. We also demonstrate that caffeine-related methylxanthines including the clinically used compound, theophylline, act at the same step of the HIV-1 life-cycle as caffeine and efficiently inhibit HIV-1 replication in primary human cells. These data reveal the feasibility of therapeutic approaches targeting host cell proteins and further support the hypothesis that ATR and ATM proteins are involved in retroviral DNA integration.
Front. Immunol., 07 July 2020 | https://doi.org/10.3389/fimmu.2020.01623
Potential Fast COVID-19 Containment With Trehalose
Daisy Martinon1, Vanessa F. Borges1, Angela C. Gomez1 and Kenichi Shimada1,2*
Countries worldwide have confirmed a staggering number of COVID-19 cases, and it is now clear that no country is immune to the SARS-CoV-2 infection. Resource-poor countries with weaker health systems are struggling with epidemics of their own and are now in a more uncertain situation with this rapidly spreading infection. Frontline healthcare workers are succumbing to the infection in their efforts to save lives. There is an urgency to develop treatments for COVID-19, yet there is limited clinical data on the efficacy of potential drug treatments. Countries worldwide implemented a stay-at-home order to “flatten the curve” and relieve the pressure on the health system, but it is uncertain how this will unfold after the economy reopens. Trehalose, a natural glucose disaccharide, is known to impair viral function through the autophagy system. Here, we propose trehalose as a potential preventative treatment for SARS-CoV-2 infection and transmission.
Antimicrob Agents Chemother. 2014 Oct; 58(10): 6315–6319.
doi: 10.1128/AAC.03420-14
Agmatine-Containing Poly(amidoamine)s as a Novel Class of Antiviral Macromolecules: Structural Properties and In Vitro Evaluation of Infectivity Inhibition
Manuela Donalisio,a Elisabetta Ranucci,b Valeria Cagno,a Andrea Civra,a Amedea Manfredi,b Roberta Cavalli,c Paolo Ferruti,b and David Lemboa
ABSTRACT
Poly(amidoamine)s (PAAs) are multifunctional tert-amine polymers endowed with high structural versatility. Here we report on the screening of a minilibrary of PAAs against a panel of viruses. The PAA AGMA1 showed antiviral activity against herpes simplex virus, human cytomegalovirus, human papillomavirus 16, and respiratory syncytial virus but not against human rotavirus and vesicular stomatitis virus. The results suggest the contribution of both a polycationic nature and side guanidine groups in imparting antiviral activity.Poly(amidoamine)s (PAAs) are multifunctional tert-amine polymers endowed with high structural versatility. Here we report on the screening of a minilibrary of PAAs against a panel of viruses. The PAA AGMA1 showed antiviral activity against herpes simplex virus, human cytomegalovirus, human papillomavirus 16, and respiratory syncytial virus but not against human rotavirus and vesicular stomatitis virus. The results suggest the contribution of both a polycationic nature and side guanidine groups in imparting antiviral activity.
PeerJ. 2020; 8: e8855.
Published online 2020 Apr 1. doi: 10.7717/peerj.8855
Prediction of antiviral drugs against African swine fever viruses based on protein–protein interaction analysis
Zhaozhong Zhu,#1 Yunshi Fan,#1 Yang Liu,2 Taijiao Jiang,3 Yang Cao,2 and Yousong Peng1
Abstract
The African swine fever virus (ASFV) has severely influenced the swine industry of the world. Unfortunately, there is currently no effective antiviral drug or vaccine against the virus. Identification of new anti-ASFV drugs is urgently needed. Here, an up-to-date set of protein–protein interactions between ASFV and swine were curated by integration of protein–protein interactions from multiple sources. Thirty-eight swine proteins were observed to interact with ASFVs and were defined as ASFV-interacting swine proteins. The ASFV-interacting swine proteins were found to play a central role in the swine protein–protein interaction network, with significant larger degree, betweenness and smaller shortest path length than other swine proteins. Some of ASFV-interacting swine proteins also interacted with several other viruses and could be taken as potential targets of drugs for broad-spectrum effect, such as HSP90AB1. Finally, the antiviral drugs which targeted ASFV-interacting swine proteins and ASFV proteins were predicted. Several drugs with either broad-spectrum effect or high specificity on ASFV-interacting swine proteins were identified, such as Polaprezinc and Geldanamycin. Structural modeling and molecular dynamics simulation showed that Geldanamycin could bind with swine HSP90AB1 stably. This work could not only deepen our understanding towards the ASFV-swine interactions, but also help for the development of effective antiviral drugs against the ASFVs.
The African swine fever virus (ASFV) has severely influenced the swine industry of the world. Unfortunately, there is currently no effective antiviral drug or vaccine against the virus. Identification of new anti-ASFV drugs is urgently needed. Here, an up-to-date set of protein–protein interactions between ASFV and swine were curated by integration of protein–protein interactions from multiple sources. Thirty-eight swine proteins were observed to interact with ASFVs and were defined as ASFV-interacting swine proteins. The ASFV-interacting swine proteins were found to play a central role in the swine protein–protein interaction network, with significant larger degree, betweenness and smaller shortest path length than other swine proteins. Some of ASFV-interacting swine proteins also interacted with several other viruses and could be taken as potential targets of drugs for broad-spectrum effect, such as HSP90AB1. Finally, the antiviral drugs which targeted ASFV-interacting swine proteins and ASFV proteins were predicted. Several drugs with either broad-spectrum effect or high specificity on ASFV-interacting swine proteins were identified, such as Polaprezinc and Geldanamycin. Structural modeling and molecular dynamics simulation showed that Geldanamycin could bind with swine HSP90AB1 stably. This work could not only deepen our understanding towards the ASFV-swine interactions, but also help for the development of effective antiviral drugs against the ASFVs.
Adv Nutr. 2019 Jul; 10(4): 696–710.
Published online 2019 Apr 22. doi: 10.1093/advances/nmz013
The Role of Zinc in Antiviral Immunity
Scott A Read,1,2 Stephanie Obeid,3 Chantelle Ahlenstiel,3 and Golo Ahlenstiel1,2
ABSTRACT
Zinc is an essential trace element that is crucial for growth, development, and the maintenance of immune function. Its influence reaches all organs and cell types, representing an integral component of approximately 10% of the human proteome, and encompassing hundreds of key enzymes and transcription factors. Zinc deficiency is strikingly common, affecting up to a quarter of the population in developing countries, but also affecting distinct populations in the developed world as a result of lifestyle, age, and disease-mediated factors. Consequently, zinc status is a critical factor that can influence antiviral immunity, particularly as zinc-deficient populations are often most at risk of acquiring viral infections such as HIV or hepatitis C virus. This review summarizes current basic science and clinical evidence examining zinc as a direct antiviral, as well as a stimulant of antiviral immunity. An abundance of evidence has accumulated over the past 50 y to demonstrate the antiviral activity of zinc against a variety of viruses, and via numerous mechanisms. The therapeutic use of zinc for viral infections such as herpes simplex virus and the common cold has stemmed from these findings; however, there remains much to be learned regarding the antiviral mechanisms and clinical benefit of zinc supplementation as a preventative and therapeutic treatment for viral infections.
Zinc is an essential trace element that is crucial for growth, development, and the maintenance of immune function. Its influence reaches all organs and cell types, representing an integral component of approximately 10% of the human proteome, and encompassing hundreds of key enzymes and transcription factors. Zinc deficiency is strikingly common, affecting up to a quarter of the population in developing countries, but also affecting distinct populations in the developed world as a result of lifestyle, age, and disease-mediated factors. Consequently, zinc status is a critical factor that can influence antiviral immunity, particularly as zinc-deficient populations are often most at risk of acquiring viral infections such as HIV or hepatitis C virus. This review summarizes current basic science and clinical evidence examining zinc as a direct antiviral, as well as a stimulant of antiviral immunity. An abundance of evidence has accumulated over the past 50 y to demonstrate the antiviral activity of zinc against a variety of viruses, and via numerous mechanisms. The therapeutic use of zinc for viral infections such as herpes simplex virus and the common cold has stemmed from these findings; however, there remains much to be learned regarding the antiviral mechanisms and clinical benefit of zinc supplementation as a preventative and therapeutic treatment for viral infections.
bioRxiv. 2021 Mar 25;2021.03.25.437060.
Sulforaphane exhibits in vitro and in vivo antiviral activity against pandemic SARS-CoV-2 and seasonal HCoV-OC43 coronaviruses
Alvaro A Ordonez, C Korin Bullen, Andres F Villabona-Rueda, Elizabeth A Thompson, Mitchell L Turner, Stephanie L Davis, Oliver Komm, Jonathan D Powell, Franco R D’Alessio, Robert H Yolken, Sanjay K Jain, Lorraine Jones-Brando
Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has incited a global health crisis. Currently, there are no orally available medications for prophylaxis for those exposed to SARS-CoV-2 and limited therapeutic options for those who develop COVID-19. We evaluated the antiviral activity of sulforaphane (SFN), a naturally occurring, orally available, well-tolerated, nutritional supplement present in high concentrations in cruciferous vegetables with limited side effects. SFN inhibited in vitro replication of four strains of SARS-CoV-2 as well as that of the seasonal coronavirus HCoV-OC43. Further, SFN and remdesivir interacted synergistically to inhibit coronavirus infection in vitro. Prophylactic administration of SFN to K18-hACE2 mice prior to intranasal SARS-CoV-2 infection significantly decreased the viral load in the lungs and upper respiratory tract and reduced lung injury and pulmonary pathology compared to untreated infected mice. SFN treatment diminished immune cell activation in the lungs, including significantly lower recruitment of myeloid cells and a reduction in T cell activation and cytokine production. Our results suggest that SFN is a promising treatment for prevention of coronavirus infection or treatment of early disease.
J Biomol Struct Dyn. 2020 : 1–9.
Published online 2020 May 6. doi: 10.1080/07391102.2020.1758790
Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations
- Aanouz,aA. Belhassan,aK. El-Khatabi,a T. Lakhlifi,a M. El-ldrissi,a and M. Bouachrinea,b
Abstract
The new Corona-virus, recently called the severe acute respiratory syndrome Coronavirus (SARS-CoV-2) appears for the first time in China and more precisely in Wuhan (December 2019). This disease can be fatal. Seniors, and people with other medical conditions (diabetes, heart disease…), may be more vulnerable and become seriously ill. This is why research into drugs to treat this infection remains essential in several research laboratories. Natural herbal remedies have long been the main, if not the only, remedy in the oral tradition for treating illnesses. Modern medicine has known its success thanks to traditional medicine, the effectiveness of which derives from medicinal plants. The objective of this study is to determine if the components of natural origin have an anti-viral effect and which can prevent humans from infection by this coronavirus using the most reliable method is molecular docking, which used to find the interaction between studied molecules and the protein, in our case we based on the inhibitor of Coronavirus (nCoV-2019) main protease. The results of molecular docking showed that among 67 molecules of natural origin, three molecules (Crocin, Digitoxigenin, and β-Eudesmol) are proposed as inhibitors against the coronavirus based on the energy types of interaction between these molecules and studied protein.
The new Corona-virus, recently called the severe acute respiratory syndrome Coronavirus (SARS-CoV-2) appears for the first time in China and more precisely in Wuhan (December 2019). This disease can be fatal. Seniors, and people with other medical conditions (diabetes, heart disease…), may be more vulnerable and become seriously ill. This is why research into drugs to treat this infection remains essential in several research laboratories. Natural herbal remedies have long been the main, if not the only, remedy in the oral tradition for treating illnesses. Modern medicine has known its success thanks to traditional medicine, the effectiveness of which derives from medicinal plants. The objective of this study is to determine if the components of natural origin have an anti-viral effect and which can prevent humans from infection by this coronavirus using the most reliable method is molecular docking, which used to find the interaction between studied molecules and the protein, in our case we based on the inhibitor of Coronavirus (nCoV-2019) main protease. The results of molecular docking showed that among 67 molecules of natural origin, three molecules (Crocin, Digitoxigenin, and β-Eudesmol) are proposed as inhibitors against the coronavirus based on the energy types of interaction between these molecules and studied protein.
ChemistrySelect. 2020 Jun 8;5(21):6312-6320.
Evaluation of the Inhibitory Activities of COVID-19 of Melaleuca cajuputi Oil Using Docking Simulation
Tran Thi Ai My 1, Huynh Thi Phuong Loan 1, Nguyen Thi Thanh Hai 1, Le Trung Hieu 1, Tran Thai Hoa 1, Bui Thi Phuong Thuy 2, Duong Tuan Quang 3, Nguyen Thanh Triet 4, Tran Thi Van Anh 5, Nguyen Thi Xuan Dieu 5, Nguyen Tien Trung 6, Nguyen Van Hue 7, Pham Van Tat 8, Vo Thanh Tung 9, Nguyen Thi Ai Nhung 1
Abstract
GC-MS was applied to identify 24 main substances in Melaleuca cajuputi essential oil (TA) extracted from fresh cajeput leaves through steam distilling. The inhibitory capability of active compounds in the TA from Thua Thien Hue, Vietnam over the Angiotensin-Converting Enzyme 2 (ACE2) protein in human body – the host receptor for SARS-CoV-2 and the main protease (PDB6LU7) of the SARS-CoV-2 using docking simulation has been studied herein. The results indicate that the ACE2 and PDB6LU7 proteins were strongly inhibited by 10 out of 24 compounds accounting for 70.9% in the TA. The most powerful anticoronavirus activity is expressed in the order: Terpineol (TA2) ≈ Guaiol (TA5) ≈ Linalool (TA19) > Cineol (TA1) > β-Selinenol (TA3) > α-Eudesmol (TA4) > γ-Eudesmol (TA7). Interestingly, the synergistic interactions of these 10 substances of the TA exhibit excellent inhibition into the ACE2 and PDB6LU7 proteins. The docking results orient that the natural Melaleuca cajuputi essential oil is considered as a valuable resource for preventing SARS-CoV-2 invasion into human body.
Molecules. 2014 Sep; 19(9): 14862–14878.
Published online 2014 Sep 17. doi: 10.3390/molecules190914862
Hinokinin, an Emerging Bioactive Lignan
Maria Carla Marcotullio,* Azzurra Pelosi, and Massimo Curini
Abstract
Hinokinin is a lignan isolated from several plant species that has been recently investigated in order to establish its biological activities. So far, its cytotoxicity, its anti-inflammatory and antimicrobial activities have been studied. Particularly interesting is its notable anti-trypanosomal activity.
4.5. Antiviral Activity
Several research groups studied the antiviral properties of hinokinin against human hepatitis B virus (HBV) [97], human immunodeficiency virus (HIV) [29], SARS-virus (SARS-CoV) [98], and in all cases 1 showed significant antiviral activity.
Hinokinin is a lignan isolated from several plant species that has been recently investigated in order to establish its biological activities. So far, its cytotoxicity, its anti-inflammatory and antimicrobial activities have been studied. Particularly interesting is its notable anti-trypanosomal activity.
Antiviral Research,. Volume 187, March 2021, 104976
First report of antiviral activity of nordihydroguaiaretic acid against Fort Sherman virus (Orthobunyavirus)
FlorenciaMartinezabMaría LauraMugasbcdJuan JavierAguilaraJulianaMarionibcdMarta SilviaContigianiaSusana C.Núñez MontoyabcdBrenda S.Konigheimab
https://doi.org/10.1016/j.antiviral.2020.104976Get rights and content
Highlights
First report of antiviral activity of nordihydroguaiaretic acid against Fort Sherman virus (Orthobunyavirus).
NDGA as a potential treatment for Orthobunyavirus.
The antiviral activity of NDGA may be due to different actions, where the SREBP and 5-LOX pathways may be involved.
NDGA would act through intracellular mechanisms.
Abstract
The genus Orthobunyavirus are a group of viruses within arbovirus, with a zoonotic cycle, some of which could lead to human infection. A characteristic of these viruses is their lack of antiviral treatment or vaccine for its prevention. The objective of this work was to study the in vitro antiviral activity of nordihydroguaiaretic acid (NDGA), the most important active compound of Larrea divaricata Cav. (Zigophyllaceae), against Fort Sherman virus (FSV) as a model of Orthobunyavirus genus. At the same time, the effect of NDGA as a lipolytic agent on the cell cycle of this viral model was assessed. The method of reducing plaque forming units on LLC-MK2 cells was used to detect the action of NDGA on CbaAr426 and SFCrEq231 isolates of FSV. NDGA did not show virucidal effect, but it had antiviral activity with a similar inhibition in both isolates, which was dose dependent. It was established that the NDGA has a better inhibition 1-h post-internalization (p.i.), showing a different behavior in each isolate, which was dependent upon the time p.i. Since virus multiplication is dependent on host cell lipid metabolism, the antiviral effect of NDGA has been previously related to its ability to disturb the lipid metabolism, probably by interfering with the 5-lipoxigenase (5-LOX) and the sterol regulatory element-binding proteins (SREBP) pathway. We determined by using caffeic acid, a 5-LOX inhibitor, that the inhibition of this enzyme negatively affected the FSV replication; and by means of resveratrol, a SREBP1 inhibitor, it was showed that the negative regulation of this pathway only had action on the SFCrEq231 reduction. In addition, it was proved that the NDGA acts intracellularly, since it showed the ability to incorporate into LLC-MK2 cells. The information provided in this work converts the NDGA into a compound with antiviral activity in vitro against FSV (Orthobunyavirus), which can be subjected to structural modifications in the future to improve the activity.
Journal of Food Biochemistry. First published: 27 September 2020
Virtual screening for functional foods against the main protease of SARS‐CoV‐2
Jiao Wang, Xiaoli Zhang, Alejandra B. Omarini, Binglin Li
https://doi.org/10.1111/jfbc.13481
Abstract
The special attention was paid on the interaction between functional foods and the main protease of severe acute respiratory syndrome coronavirus (SARS‐CoV‐2). Here, 10,870 ligands were employed and screened by the molecular docking, which involved 12 kinds of functional foods (carbohydrates, fatty acids, phospholipids, vitamin, β‐sitosterol, flavonoids, nordihydroguaiaretic acid, curcumin, nootkatone, β‐pinene, tincturoid, betulinic acid, and their isomers/analogs/derivatives). Then, 60 ligands were obtained with the good docking affinity. Most of them belong to quercetrin and its isomers/analogs/derivatives, which also showed the highest affinity for the main protease of SARS‐CoV‐2. The dynamic simulation indicated that quercetrin‐protease and quercetrin‐analog‐protease showed the excellent stability. Compared with reported docking results, quercetrin should be the best inhibitor for the main protease of SARS‐CoV‐2. Considering the green and white tea are rich in quercetrin and its isomers/analogs/derivatives, tea and relative beverages may become a good option to regulate our metabolism and help us to overcome this special time.
Antimicrob Agents Chemother. 2017 Aug; 61(8): e00376-17.
Published online 2017 Jul 25. Prepublished online 2017 May 15. doi: 10.1128/AAC.00376-17
Antiviral Activity of Nordihydroguaiaretic Acid and Its Derivative Tetra-O-Methyl Nordihydroguaiaretic Acid against West Nile Virus and Zika Virus
Teresa Merino-Ramos, Nereida Jiménez de Oya, Juan-Carlos Saiz, and Miguel A. Martín-Acebes
ABSTRACT
Flaviviruses are positive-strand RNA viruses distributed all over the world that infect millions of people every year and for which no specific antiviral agents have been approved. These viruses include the mosquito-borne West Nile virus (WNV), which is responsible for outbreaks of meningitis and encephalitis. Considering that nordihydroguaiaretic acid (NDGA) has been previously shown to inhibit the multiplication of the related dengue virus and hepatitis C virus, we have evaluated the effect of NDGA, and its methylated derivative tetra-O-methyl nordihydroguaiaretic acid (M4N), on the infection of WNV. Both compounds inhibited the infection of WNV, likely by impairing viral replication. Since flavivirus multiplication is highly dependent on host cell lipid metabolism, the antiviral effect of NDGA has been previously related to its ability to disturb the lipid metabolism, probably by interfering with the sterol regulatory element-binding proteins (SREBP) pathway. Remarkably, we observed that other structurally unrelated inhibitors of the SREBP pathway, such as PF-429242 and fatostatin, also reduced WNV multiplication, supporting that the SREBP pathway may constitute a druggable target suitable for antiviral intervention against flavivirus infection. Moreover, treatment with NDGA, M4N, PF-429242, and fatostatin also inhibited the multiplication of the mosquito-borne flavivirus Zika virus (ZIKV), which has been recently associated with birth defects (microcephaly) and neurological disorders. Our results point to SREBP inhibitors, such as NDGA and M4N, as potential candidates for further antiviral development against medically relevant flaviviruses.
Flaviviruses are positive-strand RNA viruses distributed all over the world that infect millions of people every year and for which no specific antiviral agents have been approved. These viruses include the mosquito-borne West Nile virus (WNV), which is responsible for outbreaks of meningitis and encephalitis. Considering that nordihydroguaiaretic acid (NDGA) has been previously shown to inhibit the multiplication of the related dengue virus and hepatitis C virus, we have evaluated the effect of NDGA, and its methylated derivative tetra-O-methyl nordihydroguaiaretic acid (M4N), on the infection of WNV. Both compounds inhibited the infection of WNV, likely by impairing viral replication. Since flavivirus multiplication is highly dependent on host cell lipid metabolism, the antiviral effect of NDGA has been previously related to its ability to disturb the lipid metabolism, probably by interfering with the sterol regulatory element-binding proteins (SREBP) pathway. Remarkably, we observed that other structurally unrelated inhibitors of the SREBP pathway, such as PF-429242 and fatostatin, also reduced WNV multiplication, supporting that the SREBP pathway may constitute a druggable target suitable for antiviral intervention against flavivirus infection. Moreover, treatment with NDGA, M4N, PF-429242, and fatostatin also inhibited the multiplication of the mosquito-borne flavivirus Zika virus (ZIKV), which has been recently associated with birth defects (microcephaly) and neurological disorders. Our results point to SREBP inhibitors, such as NDGA and M4N, as potential candidates for further antiviral development against medically relevant flaviviruses.
Natural Products as Potential Leads Against Coronaviruses: Could They be Encouraging Structural Models Against SARS-CoV-2?
Ilkay Erdogan Orhan & F. Sezer Senol Deniz
Natural Products and Bioprospecting volume 10, pages171–186(2020)
Abstract
New coronavirus referred to SARS-CoV-2 has caused a worldwide pandemic (COVID-19) declared by WHO. Coronavirus disease 2019 (COVID-19) is an infectious disease with severe acute respiratory syndrome caused by coronavirus-2 (SARS-CoV-2). SARS-CoV-2 is akin to SARS-CoV, which was the causative agent of severe acute respiratory syndrome (SARS) in 2002 as well as to that of Middle East respiratory syndrome (MERS) in 2012. SARS-CoV-2 has been revealed to belong to Coronaviridiae family as a member of β-coronaviruses. It has a positive-sense single-stranded RNA with the largest RNA genome. Since its genomic sequence has a notable similarity to that of SARS-CoV, antiviral drugs used to treat SARS and MERS are now being also applied for COVID-19 treatment. In order to combat SARS-CoV-2, many drug and vaccine development studies at experimental and clinical levels are currently conducted worldwide. In this sense, medicinal plants and the pure natural molecules isolated from plants have been reported to exhibit significant inhibitory antiviral activity against SARS-CoV and other types of coronaviruses. In the present review, plant extracts and natural molecules with the mentioned activity are discussed in order to give inspiration to researchers to take these molecules into consideration against SARS-CoV-2.
Terpenes
Wen et al. conducted a similar survey on 221 natural products from plants covering mostly terpene- and lignin-derivatives to measure their antiviral potential against SARS-CoV utilizing a Vero E6 cell-based CPE assay [44]. Among them, promising 20 compounds [ferruginol, dehydroabieta-7-one, sugiol, 8β-hydroxyabieta-9(11),13-dien-12-one, 6,7-dehydroroyleanone, pinusolidic acid, α-cadinol, hinokinin, savinin, 3β,12-diacetoxyabieta-6,8,11,13- tetraene, cedrane-3β,12-diol, betulonic acid, betulinic acid, cryptojaponol, 7β-hydroxy-deoxycryptojaponol, 4,4′-O-benzoylisolariciresinol, forskolin, curcumin, honokiol, and magnolol] were further evaluated for their inhibitory activity against of SARS-CoV 3CLpro through molecular modelling. The analysis showed that the active compounds having anti-SARS-CoV activity included abietane-type [ferruginol, dehydroabieta-7-one, sugiol, 8β-hydroxyabieta-9(11),13-dien-12-one, 6,7-dehydroroyleanone, and 3β,12-diacetoxyabieta-6,8,11,13-tetraene] and labdane-type diterpenes (pinusolidic acid and forskolin), sesquiterpenes (cedrane-3β,12-diol and α-cadinol), lupane-type triterpenes (betulinic acid), lignoids (hinokinin, savinin, 4,4′-O-benzoylisolariciresinol, honokiol, and magnolol), and curcumin. Among them, only betulinic acid (a lupane-type of triterpene, Ki = 8.2 ± 0.7 μM) (Fig. 1) and savinin (a lignan derivative, Ki = 9.1 ± 2.4 μM) had the capacity to block SARS-CoV 3CLpro on competitive basis.