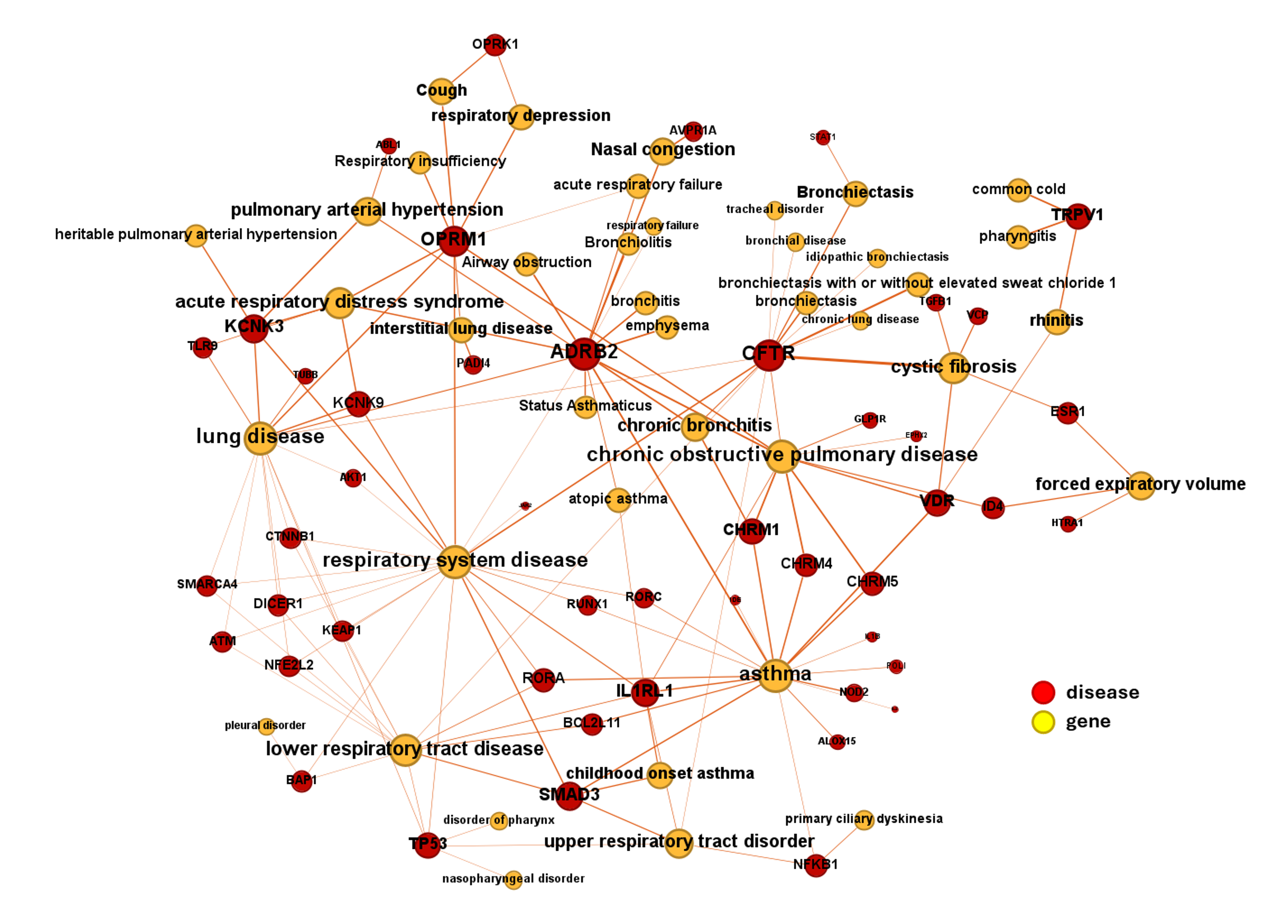
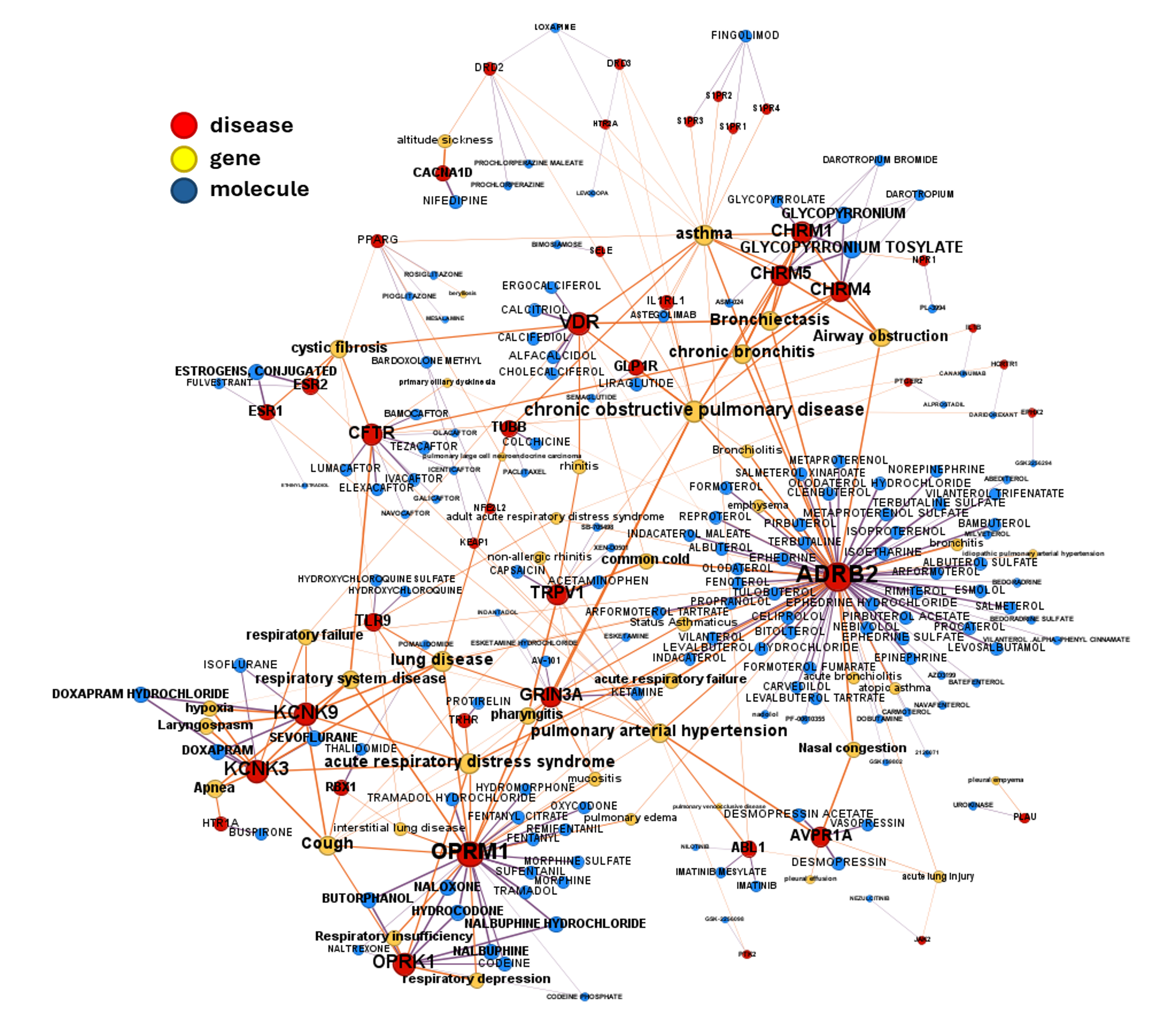
DrTarget’s AI-Powered Insights for Respiratory Diseases
DrTarget leverages advanced AI and machine learning to map target-disease associations for respiratory diseases, utilizing data from ChEMBL, Open Targets and PubChem. Our computational models uncover novel drug targets and therapeutic opportunities for conditions such as:
✔ Chronic Obstructive Pulmonary Disease (COPD)
✔ Asthma
✔ Pulmonary Fibrosis
✔ Acute Respiratory Distress Syndrome (ARDS)
✔ Cystic Fibrosis
By integrating virtual screening, drug repurposing strategies, and pathway analysis, we identify key molecular mechanisms involved in inflammation, fibrosis, and immune dysregulation. This approach supports the development of next-generation respiratory treatments, bridging the gap between AI-driven predictions and clinical research.
Check best scored target-disease associations in table:
| BioAssay Name | program | diseaseName | assayType | testedCompounds | activeCompounds | associationScore | numberOfEvidences |
|---|---|---|---|---|---|---|---|
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | chronic obstructive pulmonary disease | targetBased | 343786 | 1253 | 0.380365631777865 | 668 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | chronic obstructive pulmonary disease | targetBased | 296501 | 2737 | 0.380365631777865 | 668 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | chronic obstructive pulmonary disease | targetBased | 394050 | 3624 | 0.568331115960879 | 53 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | chronic obstructive pulmonary disease | targetBased | 335239 | 991 | 0.604623538113648 | 14 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | chronic obstructive pulmonary disease | targetBased | 335239 | 695 | 0.604623538113648 | 14 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | chronic obstructive pulmonary disease | targetBased | 405130 | 6428 | 0.429838963413524 | 38 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | chronic obstructive pulmonary disease | targetBased | 373462 | 23 | 0.429838963413524 | 38 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | chronic obstructive pulmonary disease | targetBased | 405130 | 6428 | 0.429838963413524 | 38 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | chronic obstructive pulmonary disease | targetBased | 75924 | 1804 | 0.377102675649982 | 33 |
| Novel sEH inhibitors for the therapeutic treatment of hypertension and inflammation | sEH_inhibitors | chronic obstructive pulmonary disease | targetBased | 90640 | 310 | 0.305188045034196 | 36 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | chronic obstructive pulmonary disease | targetBased | 359207 | 1189 | 0.662832074953336 | 287 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | chronic obstructive pulmonary disease | targetBased | 63676 | 1938 | 0.662832074953336 | 287 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | chronic obstructive pulmonary disease | targetBased | 359207 | 316 | 0.662832074953336 | 287 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | chronic obstructive pulmonary disease | targetBased | 63656 | 2179 | 0.662832074953336 | 287 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | chronic obstructive pulmonary disease | targetBased | 359207 | 4555 | 0.662832074953336 | 287 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | chronic obstructive pulmonary disease | targetBased | 339297 | 1446 | 0.693504127735036 | 1633 |
| SMM ID4 Measured in Biochemical System Using Small Molecule MicroArray - 2128-01_Other_SinglePoint_HTS_Activity | ID4 | chronic obstructive pulmonary disease | targetBased | 31324 | 362 | 0.483585160636221 | 8 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | chronic obstructive pulmonary disease | targetBased | 363803 | 1450 | 0.65800497078166 | 173 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | chronic obstructive pulmonary disease | targetBased | 363803 | 2629 | 0.65800497078166 | 173 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | chronic obstructive pulmonary disease | targetBased | 363803 | 502 | 0.65800497078166 | 173 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | chronic obstructive pulmonary disease | targetBased | 363803 | 698 | 0.65800497078166 | 173 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | chronic obstructive pulmonary disease | targetBased | 363803 | 2133 | 0.65800497078166 | 173 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | chronic obstructive pulmonary disease | targetBased | 363803 | 1081 | 0.65800497078166 | 173 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | emphysema | targetBased | 339297 | 1446 | 0.660629027927607 | 50 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | respiratory system disease | targetBased | 343786 | 1253 | 0.604308638464213 | 6538 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | respiratory system disease | targetBased | 296501 | 2737 | 0.604308638464213 | 6538 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | respiratory system disease | pathwayBased | 64908 | 979 | 0.378130766497915 | 53 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | respiratory system disease | pathwayBased | 64908 | 278 | 0.378130766497915 | 53 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | respiratory system disease | targetBased | 336894 | 489 | 0.284151958368056 | 417 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | respiratory system disease | targetBased | 335239 | 991 | 0.56428276068475 | 66 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | respiratory system disease | targetBased | 335239 | 695 | 0.56428276068475 | 66 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | respiratory system disease | targetBased | 75924 | 1804 | 0.474780977159598 | 114 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | respiratory system disease | targetBased | 360873 | 7438 | 0.283980323863213 | 376 |
| qHTS of Nrf2 Activators | Nrf2 activators | respiratory system disease | pathwayBased | 403871 | 1243 | 0.283980323863213 | 376 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | respiratory system disease | pathwayBased | 321427 | 201 | 0.321938078914482 | 3704 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory system disease | targetBased | 54509 | 528 | 0.321938078914482 | 3704 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory system disease | targetBased | 54513 | 338 | 0.321938078914482 | 3704 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory system disease | targetBased | 125394 | 1890 | 0.321938078914482 | 3704 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory system disease | targetBased | 124022 | 1156 | 0.321938078914482 | 3704 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | respiratory system disease | targetBased | 356517 | 1139 | 0.251138433718564 | 2504 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | respiratory system disease | pathwayBased | 7671 | 874 | 0.314260976118431 | 47 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | respiratory system disease | targetBased | 304060 | 10600 | 0.314260976118431 | 47 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | respiratory system disease | pathwayBased | 305439 | 16717 | 0.314260976118431 | 47 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | respiratory system disease | targetBased | 322361 | 619 | 0.257875014611517 | 306 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | respiratory system disease | targetBased | 215667 | 993 | 0.335425172738696 | 62 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | respiratory system disease | targetBased | 218234 | 1620 | 0.335425172738696 | 62 |
| HTS of Smad transcription factor inhibitors | SMAD3 | respiratory system disease | targetBased | 88033 | 251 | 0.557918542338559 | 323 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | respiratory system disease | targetBased | 193542 | 587 | 0.273653431359506 | 161 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | respiratory system disease | targetBased | 339297 | 1446 | 0.256338312805818 | 1242 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | respiratory system disease | targetBased | 305610 | 3794 | 0.503522045856373 | 15 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | respiratory system disease | targetBased | 339674 | 2841 | 0.57220528861961 | 170 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | pulmonary arterial hypertension | targetBased | 359207 | 1432 | 0.441191064250345 | 32 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | pulmonary arterial hypertension | targetBased | 339297 | 1446 | 0.499611061442305 | 17 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | pulmonary arterial hypertension | targetBased | 339674 | 2841 | 0.614624258622599 | 235 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | lung disease | targetBased | 343786 | 1253 | 0.293629093141759 | 713 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | lung disease | targetBased | 296501 | 2737 | 0.293629093141759 | 713 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | lung disease | targetBased | 335239 | 991 | 0.581433542946119 | 43 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | lung disease | targetBased | 335239 | 695 | 0.581433542946119 | 43 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | lung disease | targetBased | 360873 | 7438 | 0.27678487273454 | 331 |
| qHTS of Nrf2 Activators | Nrf2 activators | lung disease | pathwayBased | 403871 | 1243 | 0.27678487273454 | 331 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | lung disease | pathwayBased | 321427 | 201 | 0.304660573591682 | 3175 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lung disease | targetBased | 54509 | 528 | 0.304660573591682 | 3175 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lung disease | targetBased | 54513 | 338 | 0.304660573591682 | 3175 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lung disease | targetBased | 125394 | 1890 | 0.304660573591682 | 3175 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lung disease | targetBased | 124022 | 1156 | 0.304660573591682 | 3175 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | lung disease | targetBased | 356517 | 1139 | 0.250912353985337 | 2111 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | lung disease | targetBased | 322361 | 619 | 0.254422390952243 | 211 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | lung disease | targetBased | 339297 | 1446 | 0.468166592588468 | 641 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | lung disease | targetBased | 305610 | 3794 | 0.565673039075681 | 12 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | lung disease | targetBased | 339674 | 2841 | 0.552280221607979 | 15 |
| HCS assay for microtubule stabilizers | TUBB | lung disease | targetBased | 195821 | 1625 | 0.418360168557414 | 938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | lung disease | targetBased | 343468 | 734 | 0.407391059493617 | 117 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | interstitial lung disease | targetBased | 335239 | 991 | 0.430844594398808 | 7 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | interstitial lung disease | targetBased | 335239 | 695 | 0.430844594398808 | 7 |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of Protein Arginine Deiminase 4 (PAD4) (1536 HTS) | PADI4 | interstitial lung disease | targetBased | 326022 | 1334 | 0.542477327565812 | 37 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | forced expiratory volume | targetBased | 86095 | 1442 | 0.484277020001765 | 8 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | forced expiratory volume | targetBased | 86095 | 1151 | 0.484277020001765 | 8 |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of the HTRA serine peptidase 1 (HTRA1) | HTRA1 | forced expiratory volume | targetBased | 343467 | 1710 | 0.42621457572027 | 11 |
| SMM ID4 Measured in Biochemical System Using Small Molecule MicroArray - 2128-01_Other_SinglePoint_HTS_Activity | ID4 | forced expiratory volume | targetBased | 31324 | 362 | 0.512931148576678 | 8 |
| qHTS screen for small molecules that induce genotoxicity in human embryonic kidney (HEK293T) cells expressing luciferase-tagged ELG1 | ATAD5_inhibitors | forced expiratory volume | targetBased | 330109 | 4174 | 0.346686856422428 | 6 |
| qHTS screen for small molecules that inhibit ELG1-dependent DNA repair in human embryonic kidney (HEK293T) cells expressing luciferase-tagged ELG1 | ATAD5_inhibitors | forced expiratory volume | targetBased | 330107 | 7652 | 0.346686856422428 | 6 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | forced expiratory volume | targetBased | 331671 | 10022 | 0.29396911574075 | 6 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | common cold | targetBased | 316642 | 617 | 0.660607902407363 | 99 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | chronic bronchitis | targetBased | 343786 | 1253 | 0.444115844834956 | 80 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | chronic bronchitis | targetBased | 296501 | 2737 | 0.444115844834956 | 80 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | chronic bronchitis | targetBased | 359207 | 1189 | 0.60466648961276 | 9 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | chronic bronchitis | targetBased | 63676 | 1938 | 0.60466648961276 | 9 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | chronic bronchitis | targetBased | 359207 | 316 | 0.60466648961276 | 9 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | chronic bronchitis | targetBased | 63656 | 2179 | 0.60466648961276 | 9 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | chronic bronchitis | targetBased | 359207 | 4555 | 0.60466648961276 | 9 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | chronic bronchitis | targetBased | 339297 | 1446 | 0.650991429284571 | 41 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | lower respiratory tract disease | pathwayBased | 64908 | 979 | 0.372411203974402 | 38 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | lower respiratory tract disease | pathwayBased | 64908 | 278 | 0.372411203974402 | 38 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | lower respiratory tract disease | targetBased | 75924 | 1804 | 0.385958051745479 | 75 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | lower respiratory tract disease | targetBased | 325630 | 216 | 0.401213839254712 | 124 |
| HTS of Smad transcription factor inhibitors | SMAD3 | lower respiratory tract disease | targetBased | 88033 | 251 | 0.477251917514669 | 247 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | rhinitis | targetBased | 394050 | 3624 | 0.363313472569608 | 11 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | rhinitis | targetBased | 316642 | 617 | 0.558652137696846 | 31 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Status Asthmaticus | targetBased | 339297 | 1446 | 0.564750926143729 | 15 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | bronchitis | targetBased | 339297 | 1446 | 0.610966153644012 | 26 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | respiratory failure | targetBased | 339297 | 1446 | 0.253522525727199 | 11 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | respiratory failure | targetBased | 305610 | 3794 | 0.430510676254091 | 6 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | respiratory failure | targetBased | 339674 | 2841 | 0.430510676254091 | 6 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | respiratory depression | targetBased | 290355 | 265 | 0.410642462581269 | 35 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | respiratory depression | targetBased | 335239 | 991 | 0.554945878660219 | 261 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | respiratory depression | targetBased | 335239 | 695 | 0.554945878660219 | 261 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | atopic asthma | targetBased | 75924 | 1804 | 0.325873607329096 | 11 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | atopic asthma | targetBased | 339297 | 1446 | 0.40531047015466 | 11 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | chronic lung disease | targetBased | 343786 | 1253 | 0.284310446262139 | 534 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | chronic lung disease | targetBased | 296501 | 2737 | 0.284310446262139 | 534 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | acute respiratory distress syndrome | targetBased | 335239 | 991 | 0.59062134940304 | 12 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | acute respiratory distress syndrome | targetBased | 335239 | 695 | 0.59062134940304 | 12 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | acute respiratory distress syndrome | targetBased | 339297 | 1446 | 0.574515955536212 | 10 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | acute respiratory distress syndrome | targetBased | 305610 | 3794 | 0.569651415559999 | 9 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | acute respiratory distress syndrome | targetBased | 339674 | 2841 | 0.569651415559999 | 9 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | acute respiratory distress syndrome | targetBased | 343468 | 734 | 0.370796899415496 | 8 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | adult onset asthma | targetBased | 75924 | 1804 | 0.469663335548261 | 6 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | bronchial disease | targetBased | 343786 | 1253 | 0.27824319097566 | 409 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | bronchial disease | targetBased | 296501 | 2737 | 0.27824319097566 | 409 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | adult onset asthma | targetBased | 215667 | 993 | 0.280819212097695 | 6 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | adult onset asthma | targetBased | 218234 | 1620 | 0.280819212097695 | 6 |
| HTS of Smad transcription factor inhibitors | SMAD3 | adult onset asthma | targetBased | 88033 | 251 | 0.487600993685792 | 6 |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | Nasal congestion | targetBased | 324747 | 813 | 0.619285523949174 | 12 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Nasal congestion | targetBased | 339297 | 1446 | 0.593396752228544 | 8 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Respiratory insufficiency | targetBased | 335239 | 991 | 0.560128487470336 | 8 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Respiratory insufficiency | targetBased | 335239 | 695 | 0.560128487470336 | 8 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | Apnea | targetBased | 339674 | 2841 | 0.507493179017352 | 6 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Bronchiectasis | targetBased | 343786 | 1253 | 0.523752880488721 | 210 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Bronchiectasis | targetBased | 296501 | 2737 | 0.523752880488721 | 210 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | Bronchiectasis | pathwayBased | 195980 | 5134 | 0.377339646715432 | 15 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | Bronchiectasis | targetBased | 195980 | 695 | 0.377339646715432 | 15 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Bronchiectasis | targetBased | 339297 | 1446 | 0.540316289148883 | 6 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | Cough | targetBased | 290355 | 265 | 0.516525873530802 | 9 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Cough | targetBased | 335239 | 991 | 0.581380049750662 | 12 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Cough | targetBased | 335239 | 695 | 0.581380049750662 | 12 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Bronchiolitis | targetBased | 339297 | 1446 | 0.600348864114163 | 17 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Airway obstruction | targetBased | 339297 | 1446 | 0.66063970750587 | 92 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | acute respiratory failure | targetBased | 335239 | 991 | 0.257854355545723 | 7 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | acute respiratory failure | targetBased | 335239 | 695 | 0.257854355545723 | 7 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | acute respiratory failure | targetBased | 339297 | 1446 | 0.469631009806937 | 10 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | pharyngitis | targetBased | 316642 | 617 | 0.65652647227677 | 59 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | bronchiectasis | targetBased | 343786 | 1253 | 0.604141117825392 | 510 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | bronchiectasis | targetBased | 296501 | 2737 | 0.604141117825392 | 510 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | upper respiratory tract disorder | pathwayBased | 193265 | 3100 | 0.351531774174647 | 437 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | upper respiratory tract disorder | pathwayBased | 359244 | 3094 | 0.351531774174647 | 437 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | upper respiratory tract disorder | targetBased | 75924 | 1804 | 0.355259888309021 | 39 |
| HTS of Smad transcription factor inhibitors | SMAD3 | upper respiratory tract disorder | targetBased | 88033 | 251 | 0.498401279501692 | 44 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | asthma | pathwayBased | 64908 | 979 | 0.500776184626738 | 37 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | asthma | pathwayBased | 64908 | 278 | 0.500776184626738 | 37 |
| qHTS for Inhibitors of Polymerase Iota | POLI | asthma | targetBased | 386063 | 3989 | 0.340473522407503 | 10 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | asthma | pathwayBased | 193265 | 3100 | 0.298390830613076 | 55 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | asthma | pathwayBased | 359244 | 3094 | 0.298390830613076 | 55 |
| qHTS of IL-2 Activators | IL2 | asthma | targetBased | 364617 | 238 | 0.254125861322171 | 53 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | asthma | targetBased | 394050 | 3624 | 0.631902928952217 | 41 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | asthma | targetBased | 75924 | 1804 | 0.558542526045273 | 104 |
| Fluorescence polarization-based cell-based primary high throughput screening assay to identify activators of insulin-degrading enzyme (IDE) | IDE | asthma | targetBased | 335239 | 245 | 0.280786565585466 | 11 |
| Fluorescence polarization-based cell-based primary high throughput screening assay to identify inhibitors of insulin-degrading enzyme (IDE) | IDE | asthma | targetBased | 324747 | 1316 | 0.280786565585466 | 11 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | asthma | pathwayBased | 362051 | 17187 | 0.294370986515124 | 39 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | asthma | pathwayBased | 7671 | 874 | 0.348234913278362 | 8 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | asthma | targetBased | 304060 | 10600 | 0.348234913278362 | 8 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | asthma | pathwayBased | 305439 | 16717 | 0.348234913278362 | 8 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | asthma | targetBased | 215667 | 993 | 0.317840642239847 | 23 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | asthma | targetBased | 218234 | 1620 | 0.317840642239847 | 23 |
| qHTS Assay for Inhibitors of 15-hLO (15-human lipoxygenase) | ALOX15_inhibitors | asthma | targetBased | 73174 | 1034 | 0.391291961194972 | 7 |
| HTS of Smad transcription factor inhibitors | SMAD3 | asthma | targetBased | 88033 | 251 | 0.571802370116916 | 77 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | asthma | targetBased | 292323 | 1836 | 0.520166802217511 | 12 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | asthma | targetBased | 359207 | 1189 | 0.59019417757692 | 24 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | asthma | targetBased | 63676 | 1938 | 0.59019417757692 | 24 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | asthma | targetBased | 359207 | 316 | 0.59019417757692 | 24 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | asthma | targetBased | 63656 | 2179 | 0.59019417757692 | 24 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | asthma | targetBased | 359207 | 4555 | 0.59019417757692 | 24 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | asthma | targetBased | 339297 | 1446 | 0.672561601153222 | 1631 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | asthma | targetBased | 363803 | 1450 | 0.585061000347239 | 16 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | asthma | targetBased | 363803 | 2629 | 0.585061000347239 | 16 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | asthma | targetBased | 363803 | 502 | 0.585061000347239 | 16 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | asthma | targetBased | 363803 | 698 | 0.585061000347239 | 16 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | asthma | targetBased | 363803 | 2133 | 0.585061000347239 | 16 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | asthma | targetBased | 363803 | 1081 | 0.585061000347239 | 16 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | childhood onset asthma | pathwayBased | 64908 | 979 | 0.417970276977229 | 6 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | childhood onset asthma | pathwayBased | 64908 | 278 | 0.417970276977229 | 6 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | childhood onset asthma | targetBased | 75924 | 1804 | 0.48807545126411 | 27 |
| HTS of Smad transcription factor inhibitors | SMAD3 | childhood onset asthma | targetBased | 88033 | 251 | 0.528510372937196 | 12 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | asthma | targetBased | 325630 | 216 | 0.491425618907316 | 24 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | bronchiectasis with or without elevated sweat chloride 1 | targetBased | 343786 | 1253 | 0.760900509374716 | 894 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | bronchiectasis with or without elevated sweat chloride 1 | targetBased | 296501 | 2737 | 0.760900509374716 | 894 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | cystic fibrosis | targetBased | 343786 | 1253 | 0.996125989381412 | 17187 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | cystic fibrosis | targetBased | 296501 | 2737 | 0.996125989381412 | 17187 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | cystic fibrosis | targetBased | 86095 | 1442 | 0.425481493464507 | 15 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | cystic fibrosis | targetBased | 86095 | 1151 | 0.425481493464507 | 15 |
| qHTS for Inhibitors of TGF-b | TGFB1 | cystic fibrosis | pathwayBased | 403345 | 4970 | 0.480569637759883 | 159 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | cystic fibrosis | targetBased | 394050 | 3624 | 0.52252841740507 | 12 |
| Primary biochemical high-throughput screening assay to measure P97 ATPase inhibition | VCP | cystic fibrosis | targetBased | 217959 | 923 | 0.509487978883046 | 12 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | heritable pulmonary arterial hypertension | targetBased | 339674 | 2841 | 0.550807387763399 | 108 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | primary ciliary dyskinesia | pathwayBased | 193265 | 3100 | 0.371648354277948 | 20 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | primary ciliary dyskinesia | pathwayBased | 359244 | 3094 | 0.371648354277948 | 20 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | idiopathic bronchiectasis | targetBased | 343786 | 1253 | 0.281231933265529 | 310 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | idiopathic bronchiectasis | targetBased | 296501 | 2737 | 0.281231933265529 | 310 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | acute bronchiolitis | targetBased | 339297 | 1446 | 0.51000069558534 | 6 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | respiratory system disease | targetBased | 217959 | 2390 | 0.267305170653675 | 225 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | respiratory system disease | targetBased | 344733 | 7043 | 0.262064681773521 | 163 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | respiratory system disease | targetBased | 71016 | 346 | 0.285387508742238 | 105 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | respiratory system disease | targetBased | 46715 | 2829 | 0.278969248284901 | 179 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | lung disease | targetBased | 46715 | 2829 | 0.278306948306896 | 167 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | lung disease | targetBased | 336894 | 489 | 0.260711484934816 | 385 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | lung disease | targetBased | 344733 | 7043 | 0.258042393556257 | 150 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | lung disease | targetBased | 193542 | 587 | 0.266912019273606 | 136 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | lower respiratory tract disease | targetBased | 343786 | 1253 | 0.280987278872548 | 635 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | lower respiratory tract disease | targetBased | 296501 | 2737 | 0.280987278872548 | 635 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | lower respiratory tract disease | targetBased | 336894 | 489 | 0.26180507540306 | 387 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | lower respiratory tract disease | targetBased | 46715 | 2829 | 0.278407482230726 | 170 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | lower respiratory tract disease | targetBased | 344733 | 7043 | 0.259526998877001 | 153 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | lower respiratory tract disease | targetBased | 360873 | 7438 | 0.264884661357852 | 328 |
| qHTS of Nrf2 Activators | Nrf2 activators | lower respiratory tract disease | pathwayBased | 403871 | 1243 | 0.264884661357852 | 328 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | lower respiratory tract disease | pathwayBased | 321427 | 201 | 0.302669451002373 | 3281 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lower respiratory tract disease | targetBased | 54509 | 528 | 0.302669451002373 | 3281 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lower respiratory tract disease | targetBased | 54513 | 338 | 0.302669451002373 | 3281 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lower respiratory tract disease | targetBased | 125394 | 1890 | 0.302669451002373 | 3281 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | lower respiratory tract disease | targetBased | 124022 | 1156 | 0.302669451002373 | 3281 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | lower respiratory tract disease | targetBased | 322361 | 619 | 0.254278231517585 | 218 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | lower respiratory tract disease | targetBased | 71016 | 346 | 0.269982248718816 | 69 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | lower respiratory tract disease | targetBased | 193542 | 587 | 0.268537199086942 | 138 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | pleural disorder | targetBased | 71016 | 346 | 0.253869843213445 | 53 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | tracheal disorder | targetBased | 343786 | 1253 | 0.27791994371107 | 497 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | tracheal disorder | targetBased | 296501 | 2737 | 0.27791994371107 | 497 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | nasopharyngeal disorder | pathwayBased | 321427 | 201 | 0.270001228515148 | 33 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | nasopharyngeal disorder | targetBased | 54509 | 528 | 0.270001228515148 | 33 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | nasopharyngeal disorder | targetBased | 54513 | 338 | 0.270001228515148 | 33 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | nasopharyngeal disorder | targetBased | 125394 | 1890 | 0.270001228515148 | 33 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | nasopharyngeal disorder | targetBased | 124022 | 1156 | 0.270001228515148 | 33 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | upper respiratory tract disorder | targetBased | 343786 | 1253 | 0.277921987826351 | 519 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | upper respiratory tract disorder | targetBased | 296501 | 2737 | 0.277921987826351 | 519 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | upper respiratory tract disorder | pathwayBased | 321427 | 201 | 0.283124799711998 | 333 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper respiratory tract disorder | targetBased | 54509 | 528 | 0.283124799711998 | 333 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper respiratory tract disorder | targetBased | 54513 | 338 | 0.283124799711998 | 333 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper respiratory tract disorder | targetBased | 125394 | 1890 | 0.283124799711998 | 333 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper respiratory tract disorder | targetBased | 124022 | 1156 | 0.283124799711998 | 333 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | disorder of pharynx | pathwayBased | 321427 | 201 | 0.277382427343331 | 95 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of pharynx | targetBased | 54509 | 528 | 0.277382427343331 | 95 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of pharynx | targetBased | 54513 | 338 | 0.277382427343331 | 95 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of pharynx | targetBased | 125394 | 1890 | 0.277382427343331 | 95 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of pharynx | targetBased | 124022 | 1156 | 0.277382427343331 | 95 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | respiratory or thoracic disease | targetBased | 343786 | 1253 | 0.332808758011083 | 6518 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | respiratory or thoracic disease | targetBased | 296501 | 2737 | 0.332808758011083 | 6518 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | respiratory or thoracic disease | targetBased | 336894 | 489 | 0.274632786857521 | 413 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | respiratory or thoracic disease | targetBased | 217959 | 2390 | 0.267305170653675 | 225 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | respiratory or thoracic disease | targetBased | 46715 | 2829 | 0.278969248284901 | 179 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | respiratory or thoracic disease | targetBased | 360873 | 7438 | 0.270135376355121 | 370 |
| qHTS of Nrf2 Activators | Nrf2 activators | respiratory or thoracic disease | pathwayBased | 403871 | 1243 | 0.270135376355121 | 370 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | respiratory or thoracic disease | pathwayBased | 321427 | 201 | 0.31595798980029 | 3696 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory or thoracic disease | targetBased | 54509 | 528 | 0.31595798980029 | 3696 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory or thoracic disease | targetBased | 54513 | 338 | 0.31595798980029 | 3696 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory or thoracic disease | targetBased | 125394 | 1890 | 0.31595798980029 | 3696 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | respiratory or thoracic disease | targetBased | 124022 | 1156 | 0.31595798980029 | 3696 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | respiratory or thoracic disease | targetBased | 322361 | 619 | 0.256252172428189 | 305 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | respiratory or thoracic disease | targetBased | 71016 | 346 | 0.285387508742238 | 105 |
| HTS of Smad transcription factor inhibitors | SMAD3 | respiratory or thoracic disease | targetBased | 88033 | 251 | 0.262004337131526 | 317 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | respiratory or thoracic disease | targetBased | 193542 | 587 | 0.272030589176179 | 160 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | respiratory or thoracic disease | targetBased | 339674 | 2841 | 0.30173159139841 | 169 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | respiratory or thoracic disease | targetBased | 344733 | 7043 | 0.262064681773521 | 163 |
Some of these associations have also gone through clinical trials, as those in the graph below.
Find clinical trials details in table. Use scroll bar or search funtion to select specific drugs, molecular targets or diseases.
| BioAssay Name | program | gene | protName | diseaseName | molname | assayMode | clinicalPhase | clinicalStatus | studyStartDate | url | score | variantEffect | directionOnTrait | studyStopReason |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROPRANOLOL | targetBased | 4 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01070225 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute respiratory distress syndrome | ISOPROTERENOL | targetBased | 4 | Recruiting | 01/10/2022 | https://clinicaltrials.gov/study/NCT05658692 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ISOPROTERENOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AB02 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ISOPROTERENOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CB01 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ISOPROTERENOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CB51 | 1 | GoF | protect | |||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Apnea | BUSPIRONE | targetBased | 3 | Terminated | 01/09/2008 | https://clinicaltrials.gov/study/NCT00746954 | 0.7 | GoF | protect | Patient recruitment and Funding inadequate to finish trial |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Apnea | BUSPIRONE | targetBased | 3 | Terminated | 01/09/2008 | https://clinicaltrials.gov/study/NCT00746954 | 0.7 | GoF | protect | Patient recruitment and Funding inadequate to finish trial |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | Cough | THALIDOMIDE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00600028 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | MORPHINE | targetBased | 4 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01236495 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | MORPHINE | targetBased | 4 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01236495 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 4 | Active, not recruiting | 15/11/2019 | https://clinicaltrials.gov/study/NCT03834363 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 4 | Active, not recruiting | 15/11/2019 | https://clinicaltrials.gov/study/NCT03834363 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 4 | Completed | 16/11/2016 | https://clinicaltrials.gov/study/NCT02429050 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 4 | Completed | 16/11/2016 | https://clinicaltrials.gov/study/NCT02429050 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 3 | Unknown status | 01/07/2014 | https://clinicaltrials.gov/study/NCT01718496 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | MORPHINE | targetBased | 3 | Unknown status | 01/07/2014 | https://clinicaltrials.gov/study/NCT01718496 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulmonary edema | MORPHINE | targetBased | 4 | Terminated | 08/04/2017 | https://clinicaltrials.gov/study/NCT02856698 | 0.5 | GoF | protect | The study was stopped because of the adverse events |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulmonary edema | MORPHINE | targetBased | 4 | Terminated | 08/04/2017 | https://clinicaltrials.gov/study/NCT02856698 | 0.5 | GoF | protect | The study was stopped because of the adverse events |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | CODEINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA04 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | CODEINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA04 | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Cough | CODEINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA04 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | NALOXONE | targetBased | 4 | Completed | 01/04/2016 | https://clinicaltrials.gov/study/NCT02885948 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | NALOXONE | targetBased | 4 | Completed | 01/04/2016 | https://clinicaltrials.gov/study/NCT02885948 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | respiratory depression | NALOXONE | targetBased | 4 | Completed | 01/04/2016 | https://clinicaltrials.gov/study/NCT02885948 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | lung disease | COLCHICINE | targetBased | 3 | Withdrawn | 01/09/1993 | https://clinicaltrials.gov/study/NCT00000577 | 0.7 | LoF | protect | Record is an ACRN grant summary & not reflective of an individual trial. All ACTs conducted by ACRN were individually registered on the PRS. |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | asthma | COLCHICINE | targetBased | 3 | Withdrawn | 01/09/1993 | https://clinicaltrials.gov/study/NCT00000577 | 0.7 | LoF | protect | Record is an ACRN grant summary & not reflective of an individual trial. All ACTs conducted by ACRN were individually registered on the PRS. |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=008c5055-4ae3-498f-a7bf-5bd00f738c6d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2354204f-0d47-4232-8ca6-f74913847ac7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=720366f1-f2d1-6dd6-7bf1-9fdd9378b073 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1f653f79-63b9-4c2b-8fe6-d7bb29d84636 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=56728a9d-ada8-4784-898a-21d639606358 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=47c61858-72ce-409b-a3af-a63620cf7d13 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=328fd31e-e715-4aa5-a0a0-ebe64f21d8d6 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9eee551c-0bf8-4351-a0f2-68af9fc51eb7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c0b36e4d-3667-58f6-e053-2a95a90afad7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0fe37f2e-cf13-451d-8988-e96a5712e359 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 3 | Withdrawn | 01/04/2016 | https://clinicaltrials.gov/study/NCT01938144 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3658b70c-9abf-4c7e-be58-6bcc0d7c78de | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=135b2e99-92d1-4312-8ff9-ca44368f6874 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2a3f498d-445f-410b-818f-9f119bd6c492 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e902e339-b5bc-4c5d-9861-662f739ccb12 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24a562b5-501e-49ef-8c9d-27d07e86ea97 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=478e8edb-ca2c-5f76-e054-00144ff8d46c | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 3 | Completed | 01/11/2011 | https://clinicaltrials.gov/study/NCT01686646 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=251f2782-e7ae-11e1-aff1-0800200c9a66 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=28f41696-7d67-436e-aa33-86d8ba82efd7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=842706f9-eedf-1783-49ed-e6b7ae7d650e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=11c28c6c-d2f0-4092-8ef1-4262b17be50e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e8c6d31-0dec-4353-8094-f4abf92f644d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=182a105b-bd49-42b5-b4e0-46e5ec0197e3 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d3a6994-86f4-3a9a-e053-2991aa0a8a6c | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=60183922-bfd3-4377-884a-51b2f0a4c93a | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0f0f6488-0366-4bdf-ad49-cdbdced9d0c9 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e119fafe-9d50-4555-9bd9-389e4a1e2718 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=294a5ed5-8ae9-48cb-a6ec-094729ba40b2 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3fd31f1c-0fab-4fd2-a0f1-56ade1f013f0 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cdbe0b3a-ced3-465c-92a1-a526bbd07f09 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b995a0b2-898d-4a8f-b44f-097ae54e0ba5 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=27312ee4-d1f4-4a95-9c45-91fa5a01f5ba | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=028062ea-5072-45c1-80f0-e291d94b4dbe | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 3 | Not yet recruiting | 20/09/2024 | https://clinicaltrials.gov/study/NCT05070650 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bd759f58-5912-40d5-a5de-632df3e61a79 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d21e453-db6d-4c8a-b6f4-8ede319d0c95 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84e7be8f-2785-45ef-9c44-de05394fcd99 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d384e88-5c11-4600-ac6c-a38371570138 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8b253c9c-007e-4109-9d8d-2f318d522420 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dad63da3-cd1f-42a8-93d5-44b39aae1268 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42e4219a-a4ef-f090-f6a4-2fd2e213d397 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a8f798e-cd54-4b39-b8d4-28227ebbaeb5 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1241e896-be03-48f5-b825-b520189e2b3b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=15361568-74a9-4dc2-a27a-4e1298c86b51 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bc11b349-9e1c-4e3c-80d2-07749a67391e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad788820-1320-4e21-9b9f-82dc0e454335 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05e944d5-3473-4d8c-8df4-2ffc7474e9fa | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01466348 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=32b705a2-2312-46f8-871b-09df2bbff1e6 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dd372e00-c378-43bd-974c-f44f8adddb57 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9c3c12b-4efa-4ec4-a156-c403aff81753 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fc4d100b-eaa9-4e46-a27a-74094aa11d0f | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b37b786e-0cce-419d-94ed-3b64a1eb1201 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1f172d58-4050-424b-9743-130d8e618fe8 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bf30c75-e867-4a2f-a481-702d7aa96c23 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7c7dbac3-dd9e-d03a-1303-3d2a207c751b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=97c99b40-bcd9-710b-e053-2a95a90a10a7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2307f52c-8ea3-43b4-9b9f-bd60ac939f24 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1bfacd95-3300-46a3-8699-1dcca035b6df | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dd7b5ba3-652a-443f-b0b9-17abda148e67 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad6408ff-079b-4757-e053-2995a90a53eb | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=affddef4-c724-4bf6-8608-6eeffa38d684 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | common cold | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7486dadf-cd61-478c-8c0e-ad14f7e7ac6d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=523561fb-f6a7-4cde-9be2-bba02e60e208 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2354204f-0d47-4232-8ca6-f74913847ac7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=56728a9d-ada8-4784-898a-21d639606358 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ba17b25c-fc4d-4673-82a3-49ab07cab1d9 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7486dadf-cd61-478c-8c0e-ad14f7e7ac6d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=008c5055-4ae3-498f-a7bf-5bd00f738c6d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 3 | Completed | 27/09/2011 | https://clinicaltrials.gov/study/NCT01453400 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f466c660-69eb-4581-81ff-1499fa421d8b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=135b2e99-92d1-4312-8ff9-ca44368f6874 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5278a128-41ec-4494-e054-00144ff8d46c | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e94fdab-27ea-4c0f-a91c-735ce697304b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/12/2017 | https://clinicaltrials.gov/study/NCT03768882 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=11c28c6c-d2f0-4092-8ef1-4262b17be50e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0e8c6d31-0dec-4353-8094-f4abf92f644d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad6408ff-079b-4757-e053-2995a90a53eb | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e32881f5-2bb5-4756-aa86-a09473ab590b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a8f798e-cd54-4b39-b8d4-28227ebbaeb5 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=15361568-74a9-4dc2-a27a-4e1298c86b51 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4ba51b8-e86f-4227-e053-2995a90a853e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=720366f1-f2d1-6dd6-7bf1-9fdd9378b073 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7f631f45-06e4-4860-abb7-1e1b1e146035 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=491ad8a0-1d6b-457a-982a-e68b1def7b55 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=028062ea-5072-45c1-80f0-e291d94b4dbe | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4cde7fc-d86a-1418-e053-2a95a90aed25 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad788820-1320-4e21-9b9f-82dc0e454335 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=853012b8-d3b7-78d3-e053-2a91aa0ab0f2 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0f0f6488-0366-4bdf-ad49-cdbdced9d0c9 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0fe37f2e-cf13-451d-8988-e96a5712e359 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 3 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01048866 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1241e896-be03-48f5-b825-b520189e2b3b | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05e944d5-3473-4d8c-8df4-2ffc7474e9fa | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e7cf7c5d-5c9a-4201-9e30-1cd616071855 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd3f2ccc-ae74-4000-888c-7f25e41dbc3d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=763a5334-a741-4678-9947-603776622450 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24a562b5-501e-49ef-8c9d-27d07e86ea97 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b6dddc7c-370f-447b-b24e-8cc68b352c4c | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e119fafe-9d50-4555-9bd9-389e4a1e2718 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c98b5bd-be18-4334-b6c7-bf51cd8ccb00 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pharyngitis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45276fb7-80c0-4949-b59c-0970e156c13d | 1 | protect | ||||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | FENTANYL | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00696137 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | FENTANYL | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00696137 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | FENTANYL | targetBased | 3 | Completed | 01/09/2019 | https://clinicaltrials.gov/study/NCT06185127 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | FENTANYL | targetBased | 3 | Completed | 01/09/2019 | https://clinicaltrials.gov/study/NCT06185127 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL | targetBased | 4 | Completed | 20/09/2017 | https://clinicaltrials.gov/study/NCT03405090 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL | targetBased | 4 | Completed | 20/09/2017 | https://clinicaltrials.gov/study/NCT03405090 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL | targetBased | 4 | Active, not recruiting | 15/11/2019 | https://clinicaltrials.gov/study/NCT03834363 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL | targetBased | 4 | Active, not recruiting | 15/11/2019 | https://clinicaltrials.gov/study/NCT03834363 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | FENTANYL | targetBased | 4 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01368809 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | FENTANYL | targetBased | 4 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01368809 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | FENTANYL | targetBased | 4 | Not yet recruiting | 01/01/2022 | https://clinicaltrials.gov/study/NCT05024799 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | FENTANYL | targetBased | 4 | Not yet recruiting | 01/01/2022 | https://clinicaltrials.gov/study/NCT05024799 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory system disease | FENTANYL | targetBased | 4 | Recruiting | 19/06/2023 | https://clinicaltrials.gov/study/NCT05856136 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory system disease | FENTANYL | targetBased | 4 | Recruiting | 19/06/2023 | https://clinicaltrials.gov/study/NCT05856136 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial lung disease | FENTANYL | targetBased | 3 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT03018756 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial lung disease | FENTANYL | targetBased | 3 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT03018756 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | NEBIVOLOL | targetBased | 4 | Recruiting | 01/10/2021 | https://clinicaltrials.gov/study/NCT04845061 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | OXYCODONE | targetBased | 4 | Completed | 10/10/2022 | https://clinicaltrials.gov/study/NCT05598905 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory depression | OXYCODONE | targetBased | 4 | Completed | 10/10/2022 | https://clinicaltrials.gov/study/NCT05598905 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | SUFENTANIL | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | SUFENTANIL | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute respiratory distress syndrome | EPINEPHRINE | targetBased | 4 | Recruiting | 01/10/2022 | https://clinicaltrials.gov/study/NCT05658692 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Nasal congestion | EPINEPHRINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R01AA14 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | EPINEPHRINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AA01 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | EPINEPHRINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AK01 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulmonary arterial hypertension | EPINEPHRINE | targetBased | 4 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT05439460 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01705964 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e609bab7-c671-424d-bd9d-ced0affa0b4d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de6a969a-7e0f-4e5f-8d12-de5c7129d39c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=daa651e5-40e1-4e87-9775-3f7cc44dff32 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b3d99dc-cb35-4331-96cd-fedb95b96750 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a41b3e0c-8f43-49a5-90ea-0509e5fc18e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6d5af590-59f6-4f34-95ff-226a2996f985 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f03ce427-2dfa-460c-a1a9-c659d7a35b67 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74b3e639-cd9d-46b0-8505-86b0214cb20b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d785beac-3f68-4df1-926f-34da6773f295 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 3 | Completed | 01/07/2011 | https://clinicaltrials.gov/study/NCT01357642 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c07b20c-d222-4b42-87b8-9667705a2945 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 3 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01460511 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3ed35d7d-616c-43cd-9776-705821afc1f0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=da3009a0-9eed-4254-90f9-b4b024073828 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0babff47-11ed-47b6-a57d-502ca3c86973 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | Completed | 09/12/2019 | https://clinicaltrials.gov/study/NCT04207840 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=070d391a-0525-4172-add9-5cb45e0c5d67 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01737905 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 3 | Completed | 01/11/2011 | https://clinicaltrials.gov/study/NCT01476904 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd5ede55-10de-491e-a73f-17a77f89891a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3af22732-ff86-4ae2-813a-d2bcf0924471 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a01c237d-ae30-4326-9731-fde72505a4a6 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8da22eb1-0965-4d4e-b15d-91db7193548e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 4 | Unknown status | 01/01/2010 | https://clinicaltrials.gov/study/NCT00817466 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01834820 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 3 | Recruiting | 13/12/2018 | https://clinicaltrials.gov/study/NCT03567473 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01873144 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT01016249 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiolitis | EPINEPHRINE | targetBased | 4 | Completed | 01/01/2011 | https://clinicaltrials.gov/study/NCT01276821 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute bronchiolitis | EPINEPHRINE | targetBased | 4 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01871857 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL CITRATE | targetBased | 4 | Completed | 20/09/2017 | https://clinicaltrials.gov/study/NCT03405090 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | chronic obstructive pulmonary disease | FENTANYL CITRATE | targetBased | 4 | Completed | 20/09/2017 | https://clinicaltrials.gov/study/NCT03405090 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory system disease | FENTANYL CITRATE | targetBased | 4 | Recruiting | 19/06/2023 | https://clinicaltrials.gov/study/NCT05856136 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | respiratory system disease | FENTANYL CITRATE | targetBased | 4 | Recruiting | 19/06/2023 | https://clinicaltrials.gov/study/NCT05856136 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial lung disease | FENTANYL CITRATE | targetBased | 3 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT03018756 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial lung disease | FENTANYL CITRATE | targetBased | 3 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT03018756 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae35ec1f-526a-49bf-8262-c50e40190d1f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1bb952b7-8826-40a1-ac7b-38ec1bd9b94e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6dc143e7-6bab-42c8-b3e9-9d604d202e73 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ffb3297-6d69-4475-9bb3-ccecf3ede614 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d8f090fb-c591-4d6a-98b7-925b0bb17c38 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=254422f6-37e0-4dc1-ad3b-b2e20009a7b5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Unknown status | 01/05/2013 | https://clinicaltrials.gov/study/NCT01836016 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e65e8c22-d9d9-4473-8240-cfd154b8e6c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01490125 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5c2fe10-c61f-4cbe-bc45-7ab14baa691c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a90911e-adb5-4331-8d1a-f791fba549f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a56892b-7485-4570-9a1d-53ef23f07cbe | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00400153 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 18/03/2011 | https://clinicaltrials.gov/study/NCT01323621 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02103374 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cbcc9dbb-5758-4979-b84b-891f3d04f61e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021527s021lbl.pdf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=69ffacaa-c7e5-4016-9e77-79f7d724baad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478683 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Recruiting | 09/12/2020 | https://clinicaltrials.gov/study/NCT04655170 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00669617 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=acc9a53e-304d-44e4-b4ab-5303d1361118 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26138eb9-ff04-41cc-94c8-83edcba78b86 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42bfd443-1d9d-4806-b044-6ff874b92b25 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Withdrawn | 03/09/2021 | https://clinicaltrials.gov/study/NCT04446637 | 0.7 | GoF | protect | administrative decision |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3c64255e-074c-41f8-a344-3686a0685020 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Terminated | 01/10/2013 | https://clinicaltrials.gov/study/NCT01969539 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e7c1e97-b8b2-44c0-aeac-18f9142a522b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 14/07/2016 | https://clinicaltrials.gov/study/NCT02799784 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 15/10/2012 | https://clinicaltrials.gov/study/NCT01706328 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 28/09/2017 | https://clinicaltrials.gov/study/NCT03256695 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 23/07/2012 | https://clinicaltrials.gov/study/NCT01691482 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9832312e-0310-4ab6-8501-afa976fc7eb2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478696 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab1cbed4-50c4-4769-9947-1927333f278c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3d2ab1e-8f91-4a4d-aa6b-5a57f59ad56b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8e701d71-1dcb-4b84-bada-84e4f04f5e62 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a9b20e4-82e6-4151-9c9d-39aaf2a767e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0d31515-28cf-4041-9007-a5a9b52dc78d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3544356-e36a-4ce6-8235-cb0531c658ad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=953ab65b-b157-41b3-8f90-98fa9d7f20c5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0ec9932-b2a3-4776-a7e8-2aeded019da4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 23/01/2015 | https://clinicaltrials.gov/study/NCT02345161 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/02/2002 | https://clinicaltrials.gov/study/NCT00665600 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=708615b8-79b1-4eaa-b765-abb01be3233a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=071e819b-43cd-48f7-b63e-c891936dcfc4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d215fe3-1cf6-4bb3-b005-75f1a9d62235 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ec64742-5802-49f0-bbbe-95580730c0a7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29f0d08e-14d7-4659-bc00-0d84ae69cb7f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=23c05e9a-9df5-4978-8831-cf562aa4ba92 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 20/09/2017 | https://clinicaltrials.gov/study/NCT03405090 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bcc115b-88da-4ff0-b4e1-ef10d5287e54 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 15/10/2014 | https://clinicaltrials.gov/study/NCT02257385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d92c5d6b-ff10-4087-36a2-1cfc464cb967 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b49edc01-1ea6-4f88-883a-bb4bc2bf4b3f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e439746-9808-4102-a92f-bf6c3f1e3513 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Withdrawn | 01/06/2016 | https://clinicaltrials.gov/study/NCT02731846 | 0.7 | GoF | protect | It has been determined on June 10th that the 205165 study will not progress. This decision has been made prior to study start, no patients were screened. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 29/06/2016 | https://clinicaltrials.gov/study/NCT02729051 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=133fb50a-68d3-4d1b-a47a-67d07cc48da5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Unknown status | 20/06/2018 | https://clinicaltrials.gov/study/NCT03745261 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=374cbcbb-a37c-4057-b9a9-f70b3fdb803c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5329614f-ae0b-4854-bb6f-ea0b7dae87c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b72742df-1a6b-4483-aff3-3e79db5e015d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f0d280b-747e-4232-9d4d-a5c964f0daad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01019694 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=34b6ab14-9ed5-4651-9be6-84685e6aa919 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a7c031a5-3bf8-4548-8fe7-0c308a45152a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01313494 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 02/12/2020 | https://clinicaltrials.gov/study/NCT04606394 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1821db2-8a50-40e1-8448-4e59eafd2612 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00911651 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b2ff7e6-d9fb-47ed-85eb-859cb1bc8a79 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=77f24a8c-0a9a-4708-a401-3f56b5d4f2c1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f2b67032-2ad0-4db5-a8b5-f1d3943f65d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01253473 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Terminated | 01/10/2000 | https://clinicaltrials.gov/study/NCT02194205 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c53c391b-de2f-4df8-8c25-6764564580c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=16efdd16-04db-4874-92de-d1de22aee074 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=911f605d-5451-49e2-a484-00f619f2012b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=774b1ef9-015e-4e8e-9c52-d305de1ef85e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00462540 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=22367ff1-5edf-429a-955a-728e42695f60 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53176c67-ebcd-4b07-a08a-f2df3c541af9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 29/03/2018 | https://clinicaltrials.gov/study/NCT03474081 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f8839cf1-ec1c-42e6-a4d3-b089e95337e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ecc00500-58b8-491f-80df-dc6c89a08cae | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Unknown status | 11/01/2019 | https://clinicaltrials.gov/study/NCT03449056 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00359788 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 04/10/2006 | https://clinicaltrials.gov/study/NCT00388882 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=87b8cd3c-2849-4b50-b63e-9ea379165c07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cad98bce-bb10-4a04-94e7-d6f55ae60cda | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | atopic asthma | ALBUTEROL | targetBased | 3 | Completed | 09/05/2018 | https://clinicaltrials.gov/study/NCT03468790 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05f7916c-52f7-4a4f-a19b-f5e35f02fd0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=574824f3-51cc-4b94-9c10-e3204d8b19f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdda27b-e123-4f33-87e2-63f33b2b061e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d06309-0483-48c6-a9f2-b25dcc31db72 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=938ab43d-bf4c-477f-b918-aabe738d562b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0dee492-8bf9-4360-be9b-9d6ee1ecb256 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00354354 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 3 | Completed | 01/10/2002 | https://clinicaltrials.gov/study/NCT02177253 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 30/09/2014 | https://clinicaltrials.gov/study/NCT02257372 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5daaf067-49a2-4067-afa4-39411c87524f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02182856 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1c1f3881-2b68-4bdb-a12f-8134fe8ba961 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e95d573-74b2-4b0e-9397-c2cdaf7f41e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a9d01507-ae72-4322-93d5-5d857b1b2caa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cb4f1002-9372-4fac-aea3-99142f93dd37 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f248b57a-2c06-4ed8-817e-e6ff42f5ce88 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f422539-5708-46fd-944d-c88a12fc5ec3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99d6ba1a-1599-48a3-8dbd-a8b06af20fe1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=afcf38bf-b183-4569-b43f-25deb97f6693 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a1cb095-c3c6-4629-9a88-aeae949541ef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74170607-50c0-4de5-847e-64da9b1d52db | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a801d6-9573-4997-9b4a-aec6db6459ed | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=285a656c-f77f-4e77-b0a8-a0e9c901803f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29d24a6d-f9c1-4300-97c7-fec26bdbc22b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=829f381a-7888-4579-a2c5-4359764813c3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f454947c-6055-474b-bf35-29a379e01aa3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58860ee1-5a9d-4422-b9bc-78e8b7958b53 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fe2669f-ca2a-fd31-e09c-fd2819a8308c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | Completed | 23/01/2015 | https://clinicaltrials.gov/study/NCT02275052 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fbbb275-24a8-43c6-b341-baf90957cd69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute respiratory failure | ALBUTEROL | targetBased | 4 | Terminated | 01/01/2004 | https://clinicaltrials.gov/study/NCT00600639 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC02 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC02 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=667ce3ec-5217-4b10-b712-f3e54fbbf087 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29d24a6d-f9c1-4300-97c7-fec26bdbc22b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a000abb9-92a1-4c84-8932-cd40c476dc00 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1700a5b8-4029-424a-bfed-15f6737dd210 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=40f3cdbc-c9ee-4a3c-8061-a44b9a1d035c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8ee98719-c248-4373-e053-2995a90ae626 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulmonary arterial hypertension | ALBUTEROL | targetBased | 3 | Withdrawn | 01/07/2016 | https://clinicaltrials.gov/study/NCT02782052 | 0.7 | GoF | protect | Sponsor's decision |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=708615b8-79b1-4eaa-b765-abb01be3233a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Withdrawn | 01/04/2022 | https://clinicaltrials.gov/study/NCT05292976 | 0.7 | GoF | protect | Business reasons |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3c64255e-074c-41f8-a344-3686a0685020 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 15/05/2018 | https://clinicaltrials.gov/study/NCT03816267 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/08/2002 | https://clinicaltrials.gov/study/NCT00382889 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 15/10/2015 | https://clinicaltrials.gov/study/NCT02528214 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a9b20e4-82e6-4151-9c9d-39aaf2a767e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=829f381a-7888-4579-a2c5-4359764813c3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Withdrawn | 07/03/2018 | https://clinicaltrials.gov/study/NCT03363191 | 1 | GoF | protect | The study was withdrawn due to an internal decision. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8e701d71-1dcb-4b84-bada-84e4f04f5e62 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00528723 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a7c031a5-3bf8-4548-8fe7-0c308a45152a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02126839 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00384189 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab1cbed4-50c4-4769-9947-1927333f278c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ec64742-5802-49f0-bbbe-95580730c0a7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=22367ff1-5edf-429a-955a-728e42695f60 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 22/09/2018 | https://clinicaltrials.gov/study/NCT03528577 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d06309-0483-48c6-a9f2-b25dcc31db72 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 13/02/2017 | https://clinicaltrials.gov/study/NCT02969408 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5daaf067-49a2-4067-afa4-39411c87524f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3544356-e36a-4ce6-8235-cb0531c658ad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Not yet recruiting | 11/12/2023 | https://clinicaltrials.gov/study/NCT06154304 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01431950 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=87b8cd3c-2849-4b50-b63e-9ea379165c07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 09/01/2012 | https://clinicaltrials.gov/study/NCT01471340 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01907334 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01338311 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Terminated | 01/09/1998 | https://clinicaltrials.gov/study/NCT02235428 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00990847 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0ec9932-b2a3-4776-a7e8-2aeded019da4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01076322 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=16efdd16-04db-4874-92de-d1de22aee074 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00588406 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e4a92e7c-6d19-45e7-91f0-685f68383784 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 29/08/2018 | https://clinicaltrials.gov/study/NCT03562195 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01698320 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT00073827 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2013 | https://clinicaltrials.gov/study/NCT02031640 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b72742df-1a6b-4483-aff3-3e79db5e015d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00646009 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Withdrawn | 01/09/1993 | https://clinicaltrials.gov/study/NCT00000577 | 0.7 | GoF | protect | Record is an ACRN grant summary & not reflective of an individual trial. All ACTs conducted by ACRN were individually registered on the PRS. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05f7916c-52f7-4a4f-a19b-f5e35f02fd0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 27/07/2020 | https://clinicaltrials.gov/study/NCT04159519 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Unknown status | 01/09/2015 | https://clinicaltrials.gov/study/NCT02584738 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6afb1ec5-6771-4ce7-accb-fdff05ebf3b9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 10/06/2006 | https://clinicaltrials.gov/study/NCT00308685 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02141854 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1c1f3881-2b68-4bdb-a12f-8134fe8ba961 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e65e8c22-d9d9-4473-8240-cfd154b8e6c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6dc143e7-6bab-42c8-b3e9-9d604d202e73 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00487773 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29d24a6d-f9c1-4300-97c7-fec26bdbc22b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a90911e-adb5-4331-8d1a-f791fba549f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 13/10/2016 | https://clinicaltrials.gov/study/NCT02924688 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ecc00500-58b8-491f-80df-dc6c89a08cae | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00189436 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Withdrawn | 01/01/2009 | https://clinicaltrials.gov/study/NCT00830882 | 1 | GoF | protect | Lack of funding |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02139644 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Unknown status | 01/01/2009 | https://clinicaltrials.gov/study/NCT00900874 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=acc9a53e-304d-44e4-b4ab-5303d1361118 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 09/12/2019 | https://clinicaltrials.gov/study/NCT04207840 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 11/05/2016 | https://clinicaltrials.gov/study/NCT02741271 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=becf2107-bbc9-4b7b-87a5-3221ff6fc5cf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cb4f1002-9372-4fac-aea3-99142f93dd37 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 13/12/2018 | https://clinicaltrials.gov/study/NCT03769090 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a1cb095-c3c6-4629-9a88-aeae949541ef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Withdrawn | 18/01/2019 | https://clinicaltrials.gov/study/NCT03376932 | 0.7 | GoF | protect | withdrawn due to internal reasons |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 17/05/2006 | https://clinicaltrials.gov/study/NCT00363480 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 07/09/2015 | https://clinicaltrials.gov/study/NCT02502734 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01424813 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Recruiting | 30/03/2023 | https://clinicaltrials.gov/study/NCT05884970 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=071e819b-43cd-48f7-b63e-c891936dcfc4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29f0d08e-14d7-4659-bc00-0d84ae69cb7f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=34b6ab14-9ed5-4651-9be6-84685e6aa919 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2013 | https://clinicaltrials.gov/study/NCT02040766 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f248b57a-2c06-4ed8-817e-e6ff42f5ce88 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=254422f6-37e0-4dc1-ad3b-b2e20009a7b5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b2ff7e6-d9fb-47ed-85eb-859cb1bc8a79 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00646620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 31/01/2018 | https://clinicaltrials.gov/study/NCT03380429 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e7c1e97-b8b2-44c0-aeac-18f9142a522b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=374cbcbb-a37c-4057-b9a9-f70b3fdb803c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99d6ba1a-1599-48a3-8dbd-a8b06af20fe1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/10/2002 | https://clinicaltrials.gov/study/NCT00685425 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f454947c-6055-474b-bf35-29a379e01aa3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae35ec1f-526a-49bf-8262-c50e40190d1f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bcc115b-88da-4ff0-b4e1-ef10d5287e54 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00124176 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 20/03/2019 | https://clinicaltrials.gov/study/NCT03847896 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26138eb9-ff04-41cc-94c8-83edcba78b86 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00393367 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01747629 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00272753 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT00064389 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=133fb50a-68d3-4d1b-a47a-67d07cc48da5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d215fe3-1cf6-4bb3-b005-75f1a9d62235 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42bfd443-1d9d-4806-b044-6ff874b92b25 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a801d6-9573-4997-9b4a-aec6db6459ed | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a9d01507-ae72-4322-93d5-5d857b1b2caa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00073814 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1821db2-8a50-40e1-8448-4e59eafd2612 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Active, not recruiting | 09/07/2021 | https://clinicaltrials.gov/study/NCT04997304 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74170607-50c0-4de5-847e-64da9b1d52db | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00577655 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00252863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/11/2000 | https://clinicaltrials.gov/study/NCT00667407 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fbbb275-24a8-43c6-b341-baf90957cd69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Recruiting | 29/06/2021 | https://clinicaltrials.gov/study/NCT04276233 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53176c67-ebcd-4b07-a08a-f2df3c541af9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5329614f-ae0b-4854-bb6f-ea0b7dae87c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cad98bce-bb10-4a04-94e7-d6f55ae60cda | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Terminated | 01/02/2008 | https://clinicaltrials.gov/study/NCT00634829 | 0.7 | GoF | protect | IND voluntarily withdrawn, without prejudice |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e95d573-74b2-4b0e-9397-c2cdaf7f41e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=911f605d-5451-49e2-a484-00f619f2012b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0dee492-8bf9-4360-be9b-9d6ee1ecb256 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f8839cf1-ec1c-42e6-a4d3-b089e95337e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Terminated | 01/10/2010 | https://clinicaltrials.gov/study/NCT01218009 | 0.7 | GoF | protect | Change to study required. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 26/12/2013 | https://clinicaltrials.gov/study/NCT02040779 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d92c5d6b-ff10-4087-36a2-1cfc464cb967 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 22/06/2017 | https://clinicaltrials.gov/study/NCT03184987 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 25/07/2007 | https://clinicaltrials.gov/study/NCT00530062 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Terminated | 01/09/2013 | https://clinicaltrials.gov/study/NCT01951378 | 1 | GoF | protect | No data are available for this study as the PI has left the institution. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/02/2001 | https://clinicaltrials.gov/study/NCT00685126 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0d31515-28cf-4041-9007-a5a9b52dc78d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fe2669f-ca2a-fd31-e09c-fd2819a8308c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ffb3297-6d69-4475-9bb3-ccecf3ede614 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cbcc9dbb-5758-4979-b84b-891f3d04f61e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=afcf38bf-b183-4569-b43f-25deb97f6693 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/05/2006 | https://clinicaltrials.gov/study/NCT01091337 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 04/11/2004 | https://clinicaltrials.gov/study/NCT00315744 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Terminated | 01/09/2007 | https://clinicaltrials.gov/study/NCT00635505 | 0.7 | GoF | protect | IND voluntarily withdrawn, without prejudice |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Unknown status | 01/10/2006 | https://clinicaltrials.gov/study/NCT00385359 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 30/09/2015 | https://clinicaltrials.gov/study/NCT02513160 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1bb952b7-8826-40a1-ac7b-38ec1bd9b94e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9832312e-0310-4ab6-8501-afa976fc7eb2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b49edc01-1ea6-4f88-883a-bb4bc2bf4b3f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT02175771 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00861926 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=23c05e9a-9df5-4978-8831-cf562aa4ba92 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/04/2016 | https://clinicaltrials.gov/study/NCT02584257 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e439746-9808-4102-a92f-bf6c3f1e3513 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=938ab43d-bf4c-477f-b918-aabe738d562b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT04750603 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00831376 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=953ab65b-b157-41b3-8f90-98fa9d7f20c5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/05/1998 | https://clinicaltrials.gov/study/NCT02182713 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/07/1993 | https://clinicaltrials.gov/study/NCT00940927 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 17/03/2016 | https://clinicaltrials.gov/study/NCT02654145 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 05/04/2017 | https://clinicaltrials.gov/study/NCT03493503 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=574824f3-51cc-4b94-9c10-e3204d8b19f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 16/02/2021 | https://clinicaltrials.gov/study/NCT04677959 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205636s004lbl.pdf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f2b67032-2ad0-4db5-a8b5-f1d3943f65d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58860ee1-5a9d-4422-b9bc-78e8b7958b53 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Recruiting | 22/12/2023 | https://clinicaltrials.gov/study/NCT06245551 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00144846 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 12/11/2012 | https://clinicaltrials.gov/study/NCT01687296 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f0d280b-747e-4232-9d4d-a5c964f0daad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00497523 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT00273962 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00634517 | 0.7 | GoF | protect | IND voluntarily withdrawn, without prejudice |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d8f090fb-c591-4d6a-98b7-925b0bb17c38 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c53c391b-de2f-4df8-8c25-6764564580c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=285a656c-f77f-4e77-b0a8-a0e9c901803f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01096017 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=774b1ef9-015e-4e8e-9c52-d305de1ef85e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 14/11/2014 | https://clinicaltrials.gov/study/NCT02233803 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=69ffacaa-c7e5-4016-9e77-79f7d724baad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00394329 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f422539-5708-46fd-944d-c88a12fc5ec3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=77f24a8c-0a9a-4708-a401-3f56b5d4f2c1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 09/10/2015 | https://clinicaltrials.gov/study/NCT02483975 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 11/11/2021 | https://clinicaltrials.gov/study/NCT05084222 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a56892b-7485-4570-9a1d-53ef23f07cbe | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 3 | Completed | 27/04/2015 | https://clinicaltrials.gov/study/NCT02414854 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Recruiting | 20/02/2019 | https://clinicaltrials.gov/study/NCT03822637 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3d2ab1e-8f91-4a4d-aa6b-5a57f59ad56b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdda27b-e123-4f33-87e2-63f33b2b061e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5c2fe10-c61f-4cbe-bc45-7ab14baa691c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL | targetBased | 4 | Completed | 26/10/2020 | https://clinicaltrials.gov/study/NCT03890666 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | lung disease | ALBUTEROL | targetBased | 3 | Withdrawn | 01/09/1993 | https://clinicaltrials.gov/study/NCT00000577 | 0.7 | GoF | protect | Record is an ACRN grant summary & not reflective of an individual trial. All ACTs conducted by ACRN were individually registered on the PRS. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute bronchiolitis | ALBUTEROL | targetBased | 4 | Not yet recruiting | 20/07/2020 | https://clinicaltrials.gov/study/NCT03880903 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Status Asthmaticus | ALBUTEROL | targetBased | 4 | Completed | 05/04/2017 | https://clinicaltrials.gov/study/NCT03493503 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Status Asthmaticus | ALBUTEROL | targetBased | 4 | Terminated | 10/09/2018 | https://clinicaltrials.gov/study/NCT02966184 | 1 | GoF | protect | Study Drug Backorder |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | CELIPROLOL | targetBased | 4 | Completed | 07/06/2016 | https://clinicaltrials.gov/study/NCT02380053 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | CARVEDILOL | targetBased | 4 | Completed | 01/08/2012 | https://clinicaltrials.gov/study/NCT01656005 | 1 | LoF | protect | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | lung disease | KETAMINE | targetBased | 3 | Completed | 01/09/2019 | https://clinicaltrials.gov/study/NCT06185127 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | Status Asthmaticus | KETAMINE | targetBased | 3 | Withdrawn | 01/01/2020 | https://clinicaltrials.gov/study/NCT03338205 | 0.7 | LoF | protect | Unable to enroll patients | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | pharyngitis | KETAMINE | targetBased | 3 | Completed | 15/11/2018 | https://clinicaltrials.gov/study/NCT03729973 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | pharyngitis | KETAMINE | targetBased | 3 | Completed | 19/08/2021 | https://clinicaltrials.gov/study/NCT04955158 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | chronic obstructive pulmonary disease | KETAMINE | targetBased | 4 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT02962999 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | mucositis | KETAMINE | targetBased | 3 | Withdrawn | 01/08/2009 | https://clinicaltrials.gov/study/NCT00474110 | 0.7 | LoF | protect | Clinical practice had changed between time of initial protocol development and subject recruitment. We were not able to find eligible patients. | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | acute respiratory failure | KETAMINE | targetBased | 4 | Recruiting | 06/04/2022 | https://clinicaltrials.gov/study/NCT05277896 | 1 | LoF | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute respiratory distress syndrome | ESMOLOL | targetBased | 3 | Recruiting | 20/02/2023 | https://clinicaltrials.gov/study/NCT06013319 | 0.7 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ESMOLOL | targetBased | 4 | Not yet recruiting | 01/02/2023 | https://clinicaltrials.gov/study/NCT05694585 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | METAPROTERENOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=43ff5156-e08b-4dc4-93a6-8c727676daa1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | METAPROTERENOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cf566e38-f6ff-4b86-9537-8684943b36eb | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Respiratory insufficiency | BUTORPHANOL | targetBased | 4 | Not yet recruiting | 01/01/2022 | https://clinicaltrials.gov/study/NCT05024799 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | BUTORPHANOL | targetBased | 4 | Not yet recruiting | 01/01/2022 | https://clinicaltrials.gov/study/NCT05024799 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | BUTORPHANOL | targetBased | 4 | Not yet recruiting | 01/01/2022 | https://clinicaltrials.gov/study/NCT05024799 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FENOTEROL | targetBased | 3 | Completed | 01/10/2002 | https://clinicaltrials.gov/study/NCT00274066 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FENOTEROL | targetBased | 3 | Terminated | 01/04/2003 | https://clinicaltrials.gov/study/NCT02176187 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FENOTEROL | targetBased | 3 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02173782 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FENOTEROL | targetBased | 3 | Terminated | 01/04/2003 | https://clinicaltrials.gov/study/NCT02176200 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | FENOTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC04 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | FENOTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC04 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FENOTEROL | targetBased | 3 | Completed | 01/09/1998 | https://clinicaltrials.gov/study/NCT02182505 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FENOTEROL | targetBased | 3 | Completed | 01/04/1998 | https://clinicaltrials.gov/study/NCT02182479 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FENOTEROL | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01095016 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FENOTEROL | targetBased | 3 | Terminated | 01/02/1990 | https://clinicaltrials.gov/study/NCT02177370 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CALCITRIOL | targetBased | 4 | Unknown status | 01/08/2008 | https://clinicaltrials.gov/study/NCT00712205 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | CLENBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC13 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | CLENBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC14 | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB | targetBased | 3 | Completed | 20/04/2011 | https://clinicaltrials.gov/study/NCT01392469 | 0.7 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB | targetBased | 3 | Terminated | 01/06/2011 | https://clinicaltrials.gov/study/NCT01392495 | 0.7 | LoF | protect | Novartis discontinued the development of imatinib in PAH due to requirement of regulatory authorities for additional data to secure marketing approval in PAH. |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB | targetBased | 3 | Completed | 22/01/2014 | https://clinicaltrials.gov/study/NCT02042014 | 0.7 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB | targetBased | 3 | Completed | 01/09/2009 | https://clinicaltrials.gov/study/NCT00902174 | 0.7 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB | targetBased | 3 | Terminated | 01/04/2010 | https://clinicaltrials.gov/study/NCT01117987 | 0.7 | LoF | protect | Novartis discontinued the development of imatinib in PAH due to requirement of regulatory authorities for additional data to secure marketing approval in PAH. |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | non-allergic rhinitis | CAPSAICIN | targetBased | 3 | Recruiting | 10/09/2019 | https://clinicaltrials.gov/study/NCT05093478 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | non-allergic rhinitis | CAPSAICIN | targetBased | 4 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02288156 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rhinitis | CAPSAICIN | targetBased | 4 | Completed | 01/01/2011 | https://clinicaltrials.gov/study/NCT01223820 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rhinitis | CAPSAICIN | targetBased | 4 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01862523 | 1 | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | LEVOSALBUTAMOL | targetBased | 3 | Withdrawn | 03/09/2021 | https://clinicaltrials.gov/study/NCT04446637 | 0.7 | GoF | protect | administrative decision |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | LEVOSALBUTAMOL | targetBased | 3 | Active, not recruiting | 28/09/2023 | https://clinicaltrials.gov/study/NCT06040424 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | LEVOSALBUTAMOL | targetBased | 3 | Recruiting | 25/08/2023 | https://clinicaltrials.gov/study/NCT05890638 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4be7615b-70a5-4912-b4a5-09656cab2187 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 27/04/2015 | https://clinicaltrials.gov/study/NCT02414854 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT00073827 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c99a7f95-0092-48c1-9195-3431250633da | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00073814 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c4cf6b3-36d4-4b41-84e9-682012d7e4e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1700a5b8-4029-424a-bfed-15f6737dd210 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f3ce78d9-e923-400e-b3cd-3e6124ac7f6d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=91b2b443-bdc7-4045-bb94-906199125f49 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | Terminated | 01/01/2009 | https://clinicaltrials.gov/study/NCT00819637 | 1 | GoF | protect | Unable to enroll r/t study design & staffing issues. The trial terminated. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00831376 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Terminated | 01/07/2014 | https://clinicaltrials.gov/study/NCT02150499 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT02170532 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6925ab5e-4f50-4d04-b55a-8a6f74474382 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35eb525b-72a5-4ef9-a36a-531e9e6b3560 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 15/10/2015 | https://clinicaltrials.gov/study/NCT02528214 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00809757 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=322c7883-f01b-4ce9-b667-4924717a4e6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d91161c1-cae8-4d69-a37c-fb7b64b7736a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6be0f39e-eae0-4636-b1b3-1b31fe38301c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=206bbdec-04f0-40e3-a259-32fc6bce20d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | Withdrawn | 01/01/2009 | https://clinicaltrials.gov/study/NCT00830882 | 1 | GoF | protect | Lack of funding |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1e9d249-33d1-4b14-b92e-8f4dccded1da | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00585039 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT00064389 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7e2644e6-36c5-4988-8e52-bec90e2cd2f0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 3 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00073840 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1b7537a3-409e-4d10-87db-302f05ce684e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24b4489f-9e6d-4895-bf8b-a6b31050b1b2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVOSALBUTAMOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c47c47d-45f7-4eb4-b1f8-7d6c633a1f69 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | REMIFENTANIL | targetBased | 4 | Unknown status | 15/02/2019 | https://clinicaltrials.gov/study/NCT03783676 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | REMIFENTANIL | targetBased | 4 | Unknown status | 15/02/2019 | https://clinicaltrials.gov/study/NCT03783676 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | REMIFENTANIL | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00665119 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Respiratory insufficiency | REMIFENTANIL | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00665119 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | REMIFENTANIL | targetBased | 4 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00391105 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | REMIFENTANIL | targetBased | 4 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00391105 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CALCIFEDIOL | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02805907 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01248065 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00504946 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CHOLECALCIFEROL | targetBased | 4 | Terminated | 26/06/2015 | https://clinicaltrials.gov/study/NCT02424552 | 1 | GoF | protect | recruitment problems |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | CHOLECALCIFEROL | targetBased | 3 | Active, not recruiting | 01/09/2009 | https://clinicaltrials.gov/study/NCT00920621 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | cystic fibrosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01880346 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | cystic fibrosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 13/03/2020 | https://clinicaltrials.gov/study/NCT04118010 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | chronic obstructive pulmonary disease | CHOLECALCIFEROL | targetBased | 4 | Completed | 18/02/2020 | https://clinicaltrials.gov/study/NCT05431218 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | chronic obstructive pulmonary disease | CHOLECALCIFEROL | targetBased | 4 | Withdrawn | 01/09/2011 | https://clinicaltrials.gov/study/NCT01416701 | 1 | GoF | protect | It was not possible to enroll the planned amount of subjects. Many patients with chronic obstructive pulmonary disease already took large doses of vitamin D. |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | chronic obstructive pulmonary disease | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00914810 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | rhinitis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT02272101 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Bronchiectasis | CHOLECALCIFEROL | targetBased | 4 | Unknown status | 01/01/2015 | https://clinicaltrials.gov/study/NCT02507843 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | RIMITEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC05 | 1 | GoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | respiratory failure | ISOFLURANE | targetBased | 3 | Recruiting | 15/06/2020 | https://clinicaltrials.gov/study/NCT04415060 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | respiratory failure | ISOFLURANE | targetBased | 3 | Recruiting | 15/06/2020 | https://clinicaltrials.gov/study/NCT04415060 | 0.7 | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | SALMETEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | SALMETEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | SALMETEROL | targetBased | 4 | Unknown status | 01/06/2011 | https://clinicaltrials.gov/study/NCT01361984 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d9728e-6b5c-4aee-bfb0-745e542ed2e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Withdrawn | 01/08/2011 | https://clinicaltrials.gov/study/NCT01393145 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 13/06/2013 | https://clinicaltrials.gov/study/NCT01879410 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709903 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00530842 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Unknown status | 20/06/2018 | https://clinicaltrials.gov/study/NCT03745261 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3beef422-8a07-4a45-9ba6-414511e4b7e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01536587 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/02/1999 | https://clinicaltrials.gov/study/NCT02172287 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00144911 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 13/10/2015 | https://clinicaltrials.gov/study/NCT02516592 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01762800 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Terminated | 15/04/2008 | https://clinicaltrials.gov/study/NCT00662740 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 30/04/2010 | https://clinicaltrials.gov/study/NCT01110200 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Unknown status | 01/10/2016 | https://clinicaltrials.gov/study/NCT02941679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 10/01/2017 | https://clinicaltrials.gov/study/NCT02813200 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00527826 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Unknown status | 01/07/2014 | https://clinicaltrials.gov/study/NCT02546349 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00864812 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e0bdea8-df9b-4873-b24b-919cebff5edd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 29/10/2009 | https://clinicaltrials.gov/study/NCT00984659 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01245569 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00379028 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01908140 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 18/03/2011 | https://clinicaltrials.gov/study/NCT01323621 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de811b6d-ca0e-4921-a127-b10101ee0035 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00821093 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01089127 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/02/2005 | https://clinicaltrials.gov/study/NCT00269126 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/07/2008 | https://clinicaltrials.gov/study/NCT02136875 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/10/2002 | https://clinicaltrials.gov/study/NCT00268177 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00361959 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/02/1999 | https://clinicaltrials.gov/study/NCT02173691 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT01853787 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 14/08/2017 | https://clinicaltrials.gov/study/NCT03240575 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Withdrawn | 01/12/2013 | https://clinicaltrials.gov/study/NCT01860066 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT00274560 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 29/03/2017 | https://clinicaltrials.gov/study/NCT03055988 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00542880 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/02/2002 | https://clinicaltrials.gov/study/NCT00685841 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Terminated | 01/12/2006 | https://clinicaltrials.gov/study/NCT00346749 | 1 | GoF | protect | The study was terminated due to difficulties with finding sites and subjects willing to participate. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01342913 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b20c1d84-5c49-47de-bd2e-f987ff67faef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00115492 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 28/04/2004 | https://clinicaltrials.gov/study/NCT00355342 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 20/11/2015 | https://clinicaltrials.gov/study/NCT02603393 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 15/10/2012 | https://clinicaltrials.gov/study/NCT01706328 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Unknown status | 01/07/2009 | https://clinicaltrials.gov/study/NCT01243788 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00549146 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT01186653 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00975195 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01969721 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00567996 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00669617 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 15/04/2008 | https://clinicaltrials.gov/study/NCT00662792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 22/03/2018 | https://clinicaltrials.gov/study/NCT03395002 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Withdrawn | 01/12/2013 | https://clinicaltrials.gov/study/NCT01834885 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4eeb5f6a-593f-4a9e-9692-adefa2caf8fc | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/05/2008 | https://clinicaltrials.gov/study/NCT00673478 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00239499 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00274534 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01555138 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/12/2016 | https://clinicaltrials.gov/study/NCT02972476 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 14/11/2013 | https://clinicaltrials.gov/study/NCT01978145 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Unknown status | 01/05/2015 | https://clinicaltrials.gov/study/NCT02477397 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00411372 | 0.7 | GoF | protect | No subjects enrolled. Study was canceled before active |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/03/2013 | https://clinicaltrials.gov/study/NCT01817764 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | atopic asthma | SALMETEROL | targetBased | 3 | Completed | 09/05/2018 | https://clinicaltrials.gov/study/NCT03468790 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f7253f0-fbea-434f-b28f-e78f201b032c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 16/06/2017 | https://clinicaltrials.gov/study/NCT03034915 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01772134 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Terminated | 13/04/2018 | https://clinicaltrials.gov/study/NCT03363503 | 1 | GoF | protect | Adequate number of patients could not be reached in the relevant centers. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f53cb2d5-3267-401e-9393-dff8357830dd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Unknown status | 01/05/2013 | https://clinicaltrials.gov/study/NCT01836016 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Terminated | 01/04/2000 | https://clinicaltrials.gov/study/NCT00158847 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00563381 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/09/2000 | https://clinicaltrials.gov/study/NCT00268216 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 28/01/2005 | https://clinicaltrials.gov/study/NCT00269087 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 30/05/2014 | https://clinicaltrials.gov/study/NCT02055352 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02270424 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4925b2c-bde1-45fd-8109-dc75bee3b7a3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00615030 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01751113 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00239421 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00784550 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01772147 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Unknown status | 01/12/2009 | https://clinicaltrials.gov/study/NCT01131806 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 18/03/2011 | https://clinicaltrials.gov/study/NCT01323634 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT02172794 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00358358 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0bfe9d76-1b0e-47a4-831d-097168d66577 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 19/07/2010 | https://clinicaltrials.gov/study/NCT01124422 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/05/2006 | https://clinicaltrials.gov/study/NCT00525564 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00633217 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00876694 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 4 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01344655 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00622635 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Completed | 04/04/2013 | https://clinicaltrials.gov/study/NCT01822899 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL | targetBased | 3 | Terminated | 15/04/2008 | https://clinicaltrials.gov/study/NCT00668772 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | SALMETEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC12 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00156819 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00327353 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Recruiting | 30/01/2023 | https://clinicaltrials.gov/study/NCT05664061 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01696214 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00370591 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/11/2005 | https://clinicaltrials.gov/study/NCT00291382 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dfaca6f9-3277-47b2-319d-1377917cb54c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00395304 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00901368 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Terminated | 08/11/2004 | https://clinicaltrials.gov/study/NCT00273026 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00262587 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b20c1d84-5c49-47de-bd2e-f987ff67faef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00200967 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01437995 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00480649 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/02/2003 | https://clinicaltrials.gov/study/NCT00920543 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01194700 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00452699 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00950794 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Unknown status | 01/07/2009 | https://clinicaltrials.gov/study/NCT00914901 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT00169546 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2007 | https://clinicaltrials.gov/study/NCT00404261 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3beef422-8a07-4a45-9ba6-414511e4b7e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00127166 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT02388373 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01647646 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f53cb2d5-3267-401e-9393-dff8357830dd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/06/2005 | https://clinicaltrials.gov/study/NCT00197106 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01202097 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Terminated | 01/03/2006 | https://clinicaltrials.gov/study/NCT00471809 | 0.5 | GoF | protect | The CARE Network DSMB recommended to the NHLBI that the MARS trial be terminated, based on a futility analysis with 55 randomized children. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01181895 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00829257 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 04/11/2004 | https://clinicaltrials.gov/study/NCT00315744 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00646620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00449046 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00517634 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00296491 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01892787 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/02/2007 | https://clinicaltrials.gov/study/NCT00441441 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00102765 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/08/2010 | https://clinicaltrials.gov/study/NCT01172821 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00245570 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00452348 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 03/12/2018 | https://clinicaltrials.gov/study/NCT03756883 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00529529 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/10/2014 | https://clinicaltrials.gov/study/NCT02245672 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Withdrawn | 01/09/2015 | https://clinicaltrials.gov/study/NCT02496715 | 0.7 | GoF | protect | Development terminated. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT01324362 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0bfe9d76-1b0e-47a4-831d-097168d66577 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Terminated | 01/07/2017 | https://clinicaltrials.gov/study/NCT03269318 | 1 | GoF | protect | Change to Primary Endpoint resulted in development of new protocol |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00646009 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00479739 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00328718 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00252863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e0bdea8-df9b-4873-b24b-919cebff5edd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 29/12/2015 | https://clinicaltrials.gov/study/NCT02554786 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Recruiting | 01/10/2007 | https://clinicaltrials.gov/study/NCT01225913 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Recruiting | 23/07/2021 | https://clinicaltrials.gov/study/NCT04503460 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00461500 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT01255579 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 18/11/2005 | https://clinicaltrials.gov/study/NCT00353873 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00296530 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01274325 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 09/07/2015 | https://clinicaltrials.gov/study/NCT02446418 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02141854 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/05/2005 | https://clinicaltrials.gov/study/NCT00242775 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f7253f0-fbea-434f-b28f-e78f201b032c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00646594 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 30/03/2011 | https://clinicaltrials.gov/study/NCT01290874 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00448435 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/08/2010 | https://clinicaltrials.gov/study/NCT01172808 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac79cc13-4241-427c-a69b-6b00dafcef00 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Terminated | 31/08/2015 | https://clinicaltrials.gov/study/NCT02491970 | 1 | GoF | protect | Difficulty of patients enrollments |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 17/10/2018 | https://clinicaltrials.gov/study/NCT03394989 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4eeb5f6a-593f-4a9e-9692-adefa2caf8fc | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 13/10/2016 | https://clinicaltrials.gov/study/NCT02924688 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2013 | https://clinicaltrials.gov/study/NCT01795664 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02301975 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=43a27267-2f2b-410d-9c30-9cb95f35d9a1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 26/07/2005 | https://clinicaltrials.gov/study/NCT00351143 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01147848 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT01526161 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4925b2c-bde1-45fd-8109-dc75bee3b7a3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/11/2001 | https://clinicaltrials.gov/study/NCT00920959 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/05/2008 | https://clinicaltrials.gov/study/NCT00565266 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01511367 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 16/02/2021 | https://clinicaltrials.gov/study/NCT04677959 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00497237 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00708227 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00379288 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00830505 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 03/05/2005 | https://clinicaltrials.gov/study/NCT00455923 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Unknown status | 01/04/2017 | https://clinicaltrials.gov/study/NCT03461627 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00394368 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 17/05/2006 | https://clinicaltrials.gov/study/NCT00363480 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Terminated | 01/10/2007 | https://clinicaltrials.gov/study/NCT00503009 | 1 | GoF | protect | lack of data |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d9728e-6b5c-4aee-bfb0-745e542ed2e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 06/11/2013 | https://clinicaltrials.gov/study/NCT01978119 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Recruiting | 02/06/2017 | https://clinicaltrials.gov/study/NCT03387241 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/11/1999 | https://clinicaltrials.gov/study/NCT00158834 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de811b6d-ca0e-4921-a127-b10101ee0035 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02113436 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 28/12/2016 | https://clinicaltrials.gov/study/NCT02980133 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00424008 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01079130 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01570478 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 08/12/2015 | https://clinicaltrials.gov/study/NCT02571777 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00102882 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01484210 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 4 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT02061280 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL | targetBased | 3 | Withdrawn | 18/01/2019 | https://clinicaltrials.gov/study/NCT03376932 | 0.7 | GoF | protect | withdrawn due to internal reasons |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | SALMETEROL | targetBased | 4 | Unknown status | 01/06/2011 | https://clinicaltrials.gov/study/NCT01361984 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | SALMETEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | SALMETEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | SALMETEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | PROCATEROL | targetBased | 4 | Not yet recruiting | 16/04/2024 | https://clinicaltrials.gov/study/NCT06346691 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | PROCATEROL | targetBased | 4 | Terminated | 01/05/2006 | https://clinicaltrials.gov/study/NCT00394485 | 1 | GoF | protect | Difficulty in recruting patients |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | PROCATEROL | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=R03AC16 | 0.7 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | PROCATEROL | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=R03CC08 | 0.7 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROCATEROL | targetBased | 4 | Completed | 01/05/2006 | https://clinicaltrials.gov/study/NCT01091337 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROCATEROL | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01095016 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROCATEROL | targetBased | 4 | Unknown status | 01/07/2010 | https://clinicaltrials.gov/study/NCT01170429 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROCATEROL | targetBased | 3 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00990847 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PROCATEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01076322 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL | targetBased | 4 | Unknown status | 01/06/2011 | https://clinicaltrials.gov/study/NCT01361984 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00754546 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00931385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00773786 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Terminated | 01/11/2014 | https://clinicaltrials.gov/study/NCT02275481 | 1 | GoF | protect | lack of enrollment |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00909779 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00571428 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00932646 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 3 | Completed | 01/02/2002 | https://clinicaltrials.gov/study/NCT00685841 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL | targetBased | 4 | Terminated | 03/10/2017 | https://clinicaltrials.gov/study/NCT03219866 | 1 | GoF | protect | Lower enrollment than Sponsor expected - Sponsor stopped study |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ARFORMOTEROL | targetBased | 4 | Terminated | 01/01/2009 | https://clinicaltrials.gov/study/NCT00819637 | 1 | GoF | protect | Unable to enroll r/t study design & staffing issues. The trial terminated. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ARFORMOTEROL | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00734318 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ARFORMOTEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00563056 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL | targetBased | 4 | Unknown status | 01/06/2011 | https://clinicaltrials.gov/study/NCT01361984 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL | targetBased | 3 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00064415 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | ARFORMOTEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Nasal congestion | EPHEDRINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R01AB05 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Nasal congestion | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1239e824-aa60-4b7f-9910-81f96ebe6c70 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Nasal congestion | EPHEDRINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R01AA03 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e4a4021c-c092-41bf-91ed-ef483fd52732 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=73b55902-273a-4734-8deb-af6eb68b5523 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=750f9a39-acdc-4abc-800e-467884a4dcea | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=46633af4-98c1-471f-9232-d719c6d8e445 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=172e970d-6773-4796-a280-3370e2f46c69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2170f081-c03f-47e8-bc63-4052c5e653fa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | common cold | EPHEDRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1239e824-aa60-4b7f-9910-81f96ebe6c70 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | pulmonary arterial hypertension | VASOPRESSIN | targetBased | 4 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT05439460 | 1 | GoF | protect | |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d9d8442a-4722-4b41-9faa-1ee853a4cc3b | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=86f040d0-a073-4fa5-8997-b2fecc4a3205 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=30d4c387-b99c-49f8-a8bd-de23fdafb739 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8ca799f1-7029-4527-9ab4-7512504bb8e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | acute respiratory distress syndrome | NOREPINEPHRINE | targetBased | 4 | Recruiting | 01/10/2022 | https://clinicaltrials.gov/study/NCT05658692 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | HYDROCODONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA03 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Cough | HYDROCODONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA03 | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Cough | HYDROCODONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R05DA03 | 1 | GoF | protect | |||
| qHTS for Small Molecule Agonists and Allosteric Enhancers of Human TRH Receptor: Primary Screen for Agonists. | TRHR | TRHR | Thyrotropin-releasing hormone receptor | acute respiratory distress syndrome | PROTIRELIN | targetBased | 3 | Completed | 01/08/1993 | https://clinicaltrials.gov/study/NCT00004778 | 0.7 | GoF | protect | |
| qHTS for Small Molecule Agonists and Allosteric Enhancers of Human TRH Receptor: Primary Screen for Enhancers | TRHR | TRHR | Thyrotropin-releasing hormone receptor | acute respiratory distress syndrome | PROTIRELIN | targetBased | 3 | Completed | 01/08/1993 | https://clinicaltrials.gov/study/NCT00004778 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | mucositis | HYDROMORPHONE | targetBased | 3 | Withdrawn | 01/08/2009 | https://clinicaltrials.gov/study/NCT00474110 | 0.7 | GoF | protect | Clinical practice had changed between time of initial protocol development and subject recruitment. We were not able to find eligible patients. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | mucositis | HYDROMORPHONE | targetBased | 3 | Withdrawn | 01/08/2009 | https://clinicaltrials.gov/study/NCT00474110 | 0.7 | GoF | protect | Clinical practice had changed between time of initial protocol development and subject recruitment. We were not able to find eligible patients. |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | altitude sickness | NIFEDIPINE | targetBased | 4 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02463357 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | lung disease | HYDROXYCHLOROQUINE | targetBased | 3 | Withdrawn | 30/04/2020 | https://clinicaltrials.gov/study/NCT04361461 | 0.7 | LoF | protect | This study was canceled before enrollment due to a decision by the Sponsor |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | acute respiratory distress syndrome | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 17/03/2020 | https://clinicaltrials.gov/study/NCT04308668 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | acute respiratory distress syndrome | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 06/04/2020 | https://clinicaltrials.gov/study/NCT04328467 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | adult acute respiratory distress syndrome | HYDROXYCHLOROQUINE | targetBased | 3 | Terminated | 17/04/2020 | https://clinicaltrials.gov/study/NCT04347980 | 0.35 | LoF | protect | ANSM RECOMMANDATION |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | respiratory failure | HYDROXYCHLOROQUINE | targetBased | 3 | Terminated | 13/05/2020 | https://clinicaltrials.gov/study/NCT04339816 | 0.35 | LoF | protect | Steering Committee decision in accordance with stopping rule 1: Emergence of new data |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | ERGOCALCIFEROL | targetBased | 4 | Unknown status | 01/03/2014 | https://clinicaltrials.gov/study/NCT02053402 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | ERGOCALCIFEROL | targetBased | 3 | Active, not recruiting | 01/10/2018 | https://clinicaltrials.gov/study/NCT03365687 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/12/2010 | https://clinicaltrials.gov/study/NCT01447173 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01730976 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | BAMBUTEROL | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01796730 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | BAMBUTEROL | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=R03CC12 | 0.7 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | BAMBUTEROL | targetBased | 4 | Unknown status | 01/06/2012 | https://clinicaltrials.gov/study/NCT02002754 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | BAMBUTEROL | targetBased | 4 | Unknown status | 01/06/2012 | https://clinicaltrials.gov/study/NCT02002754 | 1 | GoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB MESYLATE | targetBased | 3 | Completed | 22/01/2014 | https://clinicaltrials.gov/study/NCT02042014 | 0.7 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | pulmonary arterial hypertension | IMATINIB MESYLATE | targetBased | 3 | Completed | 01/09/2009 | https://clinicaltrials.gov/study/NCT00902174 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | lung disease | HYDROXYCHLOROQUINE SULFATE | targetBased | 3 | Withdrawn | 30/04/2020 | https://clinicaltrials.gov/study/NCT04361461 | 0.7 | LoF | protect | This study was canceled before enrollment due to a decision by the Sponsor |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | respiratory system disease | DOXAPRAM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R07AB01 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | lung disease | DOXAPRAM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=954859c0-a121-34d6-e053-2a95a90a1e19 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | Apnea | DOXAPRAM | targetBased | 4 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00389909 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | lung disease | DOXAPRAM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=954859c0-a121-34d6-e053-2a95a90a1e19 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | hypoxia | DOXAPRAM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02171910 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | Apnea | DOXAPRAM | targetBased | 4 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00389909 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | respiratory system disease | DOXAPRAM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R07AB01 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | hypoxia | DOXAPRAM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02171910 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | TERBUTALINE | targetBased | 4 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02103374 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | TERBUTALINE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01944033 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | TERBUTALINE | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00421122 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | TERBUTALINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC03 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | TERBUTALINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC03 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | TERBUTALINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC53 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fbf54709-5857-4dbc-9233-ebd7abff88c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4ae8fb3d-9df3-4948-bb07-6e4169521f15 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94742710-e87e-4e1c-85ee-0a2e7a77f0de | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00849095 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7474ef3d-036f-47dd-aea7-be6d1086c0e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=382dcebb-6e41-4335-818d-b5822e3fb884 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00839800 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a95bc4f-34d3-4b5e-841b-6839e1c1e0c7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/05/2005 | https://clinicaltrials.gov/study/NCT00244608 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 07/07/2014 | https://clinicaltrials.gov/study/NCT02149199 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | Unknown status | 01/07/2009 | https://clinicaltrials.gov/study/NCT00914797 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00252863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00259766 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 28/11/2014 | https://clinicaltrials.gov/study/NCT02224157 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00837967 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=613df3cb-4d55-4d12-ab08-a932481aaa0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2a4be57a-a3df-4aa5-be52-845b75e78b3b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02322788 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Terminated | 23/08/2021 | https://clinicaltrials.gov/study/NCT04705727 | 0.7 | GoF | protect | Not enough recruitment in th trial |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=879be756-991a-42c5-a381-9c8c2ad1fbdb | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cec31032-f366-4524-9e01-63146e473b2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8265c67f-55bc-40f0-9ede-3e4b4bb178f4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=352e3476-2bc4-4995-9fd9-4a8864417f1a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ed2af9a9-8bc4-4c6a-b8df-e8fc88c93d6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01096017 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | TERBUTALINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a95bc4f-34d3-4b5e-841b-6839e1c1e0c7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Status Asthmaticus | TERBUTALINE | targetBased | 4 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00124995 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | OLODATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT01696058 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00932646 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00931385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01533922 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01040689 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT02853123 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT02006732 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01e15aee-40e0-23f3-537f-c96dd63e2cb1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 01/06/2017 | https://clinicaltrials.gov/study/NCT03152149 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01040728 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 12/02/2016 | https://clinicaltrials.gov/study/NCT02629965 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 13/01/2015 | https://clinicaltrials.gov/study/NCT02296138 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02085161 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00796653 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00793624 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01040793 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 29/03/2017 | https://clinicaltrials.gov/study/NCT03055988 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 08/03/2016 | https://clinicaltrials.gov/study/NCT02683109 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01040130 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00782210 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01536262 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 08/01/2020 | https://clinicaltrials.gov/study/NCT04223843 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01431287 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00782509 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Withdrawn | 01/08/2016 | https://clinicaltrials.gov/study/NCT02812862 | 1 | GoF | protect | Rationale obsolete |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 14/08/2017 | https://clinicaltrials.gov/study/NCT03240575 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01559116 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01525615 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 28/01/2020 | https://clinicaltrials.gov/study/NCT04231214 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 4 | Completed | 01/01/2017 | https://clinicaltrials.gov/study/NCT03425617 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01964352 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT01694771 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01533935 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01969721 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01431274 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | OLODATEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC19 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | OLODATEROL | targetBased | 4 | Completed | 11/07/2016 | https://clinicaltrials.gov/study/NCT02682862 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | OLODATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | PIRBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | PIRBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC08 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PIRBUTEROL | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/19009s6lbl.pdf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | REPROTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC14 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | REPROTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC15 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | INDACATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00821093 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01727024 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 05/02/2013 | https://clinicaltrials.gov/study/NCT01794780 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00876694 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/02/2008 | https://clinicaltrials.gov/study/NCT00624286 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01089127 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00393458 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00846586 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Withdrawn | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715311 | 1 | GoF | protect | withdrawn prior to patient recruitment |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01294787 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/12/2014 | https://clinicaltrials.gov/study/NCT02665130 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Unknown status | 01/10/2015 | https://clinicaltrials.gov/study/NCT02547558 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00792805 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00622635 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT05506865 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01072448 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/onbrez-breezhaler | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00900731 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 30/05/2014 | https://clinicaltrials.gov/study/NCT02055352 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 23/03/2013 | https://clinicaltrials.gov/study/NCT02566031 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00877383 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01555138 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/03/2013 | https://clinicaltrials.gov/study/NCT01693003 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01068600 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 28/04/2015 | https://clinicaltrials.gov/study/NCT02418468 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/05/2008 | https://clinicaltrials.gov/study/NCT00677807 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01012765 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Withdrawn | 01/09/2011 | https://clinicaltrials.gov/study/NCT01377428 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01232894 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT02473237 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00794157 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01543828 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01272362 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01437748 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00845728 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00999908 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01682863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/02/2008 | https://clinicaltrials.gov/study/NCT00615459 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00620022 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00615030 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00567996 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00669617 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 15/10/2014 | https://clinicaltrials.gov/study/NCT02257385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | INDACATEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC18 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiectasis | INDACATEROL | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 25/04/2017 | https://clinicaltrials.gov/study/NCT03100500 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01079130 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT02039011 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 29/12/2015 | https://clinicaltrials.gov/study/NCT02554786 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00529529 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | INDACATEROL | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | GoF | protect | issues related to Covid-19 restrictions/shutdowns |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | INDACATEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 10/01/2017 | https://clinicaltrials.gov/study/NCT02813200 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0bfe9d76-1b0e-47a4-831d-097168d66577 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01344655 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f7253f0-fbea-434f-b28f-e78f201b032c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00975195 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b20c1d84-5c49-47de-bd2e-f987ff67faef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de811b6d-ca0e-4921-a127-b10101ee0035 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d9728e-6b5c-4aee-bfb0-745e542ed2e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 30/04/2010 | https://clinicaltrials.gov/study/NCT01110200 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01245569 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e0bdea8-df9b-4873-b24b-919cebff5edd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3beef422-8a07-4a45-9ba6-414511e4b7e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | atopic asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 09/05/2018 | https://clinicaltrials.gov/study/NCT03468790 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 3 | Withdrawn | 01/08/2011 | https://clinicaltrials.gov/study/NCT01393145 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4925b2c-bde1-45fd-8109-dc75bee3b7a3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f53cb2d5-3267-401e-9393-dff8357830dd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/02/2005 | https://clinicaltrials.gov/study/NCT00269126 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4eeb5f6a-593f-4a9e-9692-adefa2caf8fc | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4eeb5f6a-593f-4a9e-9692-adefa2caf8fc | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02113436 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00901368 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Terminated | 08/11/2004 | https://clinicaltrials.gov/study/NCT00273026 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01647646 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01484210 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b20c1d84-5c49-47de-bd2e-f987ff67faef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00452348 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d9728e-6b5c-4aee-bfb0-745e542ed2e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00394368 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c4925b2c-bde1-45fd-8109-dc75bee3b7a3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00461500 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 03/05/2005 | https://clinicaltrials.gov/study/NCT00455923 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01202097 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/03/2013 | https://clinicaltrials.gov/study/NCT01795664 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 29/12/2015 | https://clinicaltrials.gov/study/NCT02554786 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00127166 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de811b6d-ca0e-4921-a127-b10101ee0035 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac79cc13-4241-427c-a69b-6b00dafcef00 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00262587 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e0bdea8-df9b-4873-b24b-919cebff5edd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/05/2008 | https://clinicaltrials.gov/study/NCT00565266 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Unknown status | 01/04/2017 | https://clinicaltrials.gov/study/NCT03461627 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3beef422-8a07-4a45-9ba6-414511e4b7e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/10/2014 | https://clinicaltrials.gov/study/NCT02245672 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f7253f0-fbea-434f-b28f-e78f201b032c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01511367 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0bfe9d76-1b0e-47a4-831d-097168d66577 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00452699 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00829257 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dfaca6f9-3277-47b2-319d-1377917cb54c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 3 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01274325 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f53cb2d5-3267-401e-9393-dff8357830dd | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Recruiting | 23/07/2021 | https://clinicaltrials.gov/study/NCT04503460 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=43a27267-2f2b-410d-9c30-9cb95f35d9a1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | SALMETEROL XINAFOATE | targetBased | 4 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00102882 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | SALMETEROL XINAFOATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58ed5fef-3da7-41d2-850d-55f72efcdb78 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | VILANTEROL TRIFENATATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL TRIFENATATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL TRIFENATATE | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | GoF | protect | Continuing delays due to COVID-19 pandemic |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL TRIFENATATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96428df1-ea05-431a-98d3-1ec2c4b63878 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL TRIFENATATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96428df1-ea05-431a-98d3-1ec2c4b63878 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL TRIFENATATE | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01498679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | VILANTEROL TRIFENATATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | TULOBUTEROL | targetBased | 4 | Unknown status | 01/11/2010 | https://clinicaltrials.gov/study/NCT01465906 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | TULOBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC11 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | TULOBUTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC11 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TULOBUTEROL | targetBased | 4 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00950794 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | VILANTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316900 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01716520 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478696 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 04/03/2011 | https://clinicaltrials.gov/study/NCT01336608 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 25/09/2009 | https://clinicaltrials.gov/study/NCT01009463 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 13/03/2012 | https://clinicaltrials.gov/study/NCT01551758 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01313637 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 01/06/2017 | https://clinicaltrials.gov/study/NCT03152149 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 28/01/2014 | https://clinicaltrials.gov/study/NCT01957150 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 05/10/2009 | https://clinicaltrials.gov/study/NCT01053988 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 25/01/2011 | https://clinicaltrials.gov/study/NCT01313676 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 06/06/2023 | https://clinicaltrials.gov/study/NCT04923347 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 30/06/2011 | https://clinicaltrials.gov/study/NCT01395888 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01691885 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 29/06/2016 | https://clinicaltrials.gov/study/NCT02729051 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 07/04/2014 | https://clinicaltrials.gov/study/NCT02105974 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 15/10/2012 | https://clinicaltrials.gov/study/NCT01706328 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 11/04/2018 | https://clinicaltrials.gov/study/NCT03467425 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 14/10/2022 | https://clinicaltrials.gov/study/NCT05535972 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01627327 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 16/03/2011 | https://clinicaltrials.gov/study/NCT01323660 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 18/03/2011 | https://clinicaltrials.gov/study/NCT01323621 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 23/01/2015 | https://clinicaltrials.gov/study/NCT02345161 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96428df1-ea05-431a-98d3-1ec2c4b63878 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/03/2013 | https://clinicaltrials.gov/study/NCT01817764 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01328444 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01342913 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 28/07/2015 | https://clinicaltrials.gov/study/NCT02487446 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01376245 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478683 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 18/03/2011 | https://clinicaltrials.gov/study/NCT01323634 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 25/09/2009 | https://clinicaltrials.gov/study/NCT01017952 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 23/01/2013 | https://clinicaltrials.gov/study/NCT01777334 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 19/10/2009 | https://clinicaltrials.gov/study/NCT01054885 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT02014480 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 19/09/2017 | https://clinicaltrials.gov/study/NCT05342558 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 04/04/2013 | https://clinicaltrials.gov/study/NCT01822899 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | GoF | protect | Continuing delays due to COVID-19 pandemic |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 27/07/2015 | https://clinicaltrials.gov/study/NCT02487498 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 15/09/2014 | https://clinicaltrials.gov/study/NCT01899742 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 4 | Completed | 29/03/2018 | https://clinicaltrials.gov/study/NCT03474081 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Withdrawn | 01/06/2016 | https://clinicaltrials.gov/study/NCT02731846 | 0.7 | GoF | protect | It has been determined on June 10th that the 205165 study will not progress. This decision has been made prior to study start, no patients were screened. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | VILANTEROL | targetBased | 3 | Completed | 30/06/2014 | https://clinicaltrials.gov/study/NCT02164513 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01147848 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01181895 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02094937 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Active, not recruiting | 08/08/2022 | https://clinicaltrials.gov/study/NCT04651777 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 20/10/2017 | https://clinicaltrials.gov/study/NCT03248128 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Recruiting | 29/07/2021 | https://clinicaltrials.gov/study/NCT04937387 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01498679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Withdrawn | 18/01/2019 | https://clinicaltrials.gov/study/NCT03376932 | 0.7 | GoF | protect | withdrawn due to internal reasons |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 4 | Completed | 25/05/2016 | https://clinicaltrials.gov/study/NCT02730351 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02301975 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 20/09/2012 | https://clinicaltrials.gov/study/NCT01686633 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Withdrawn | 01/01/2012 | https://clinicaltrials.gov/study/NCT01435902 | 0.7 | GoF | protect | Study was cancelled prior to enrolling any subjects |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01134042 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 20/08/2010 | https://clinicaltrials.gov/study/NCT01165138 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 4 | Withdrawn | 07/03/2018 | https://clinicaltrials.gov/study/NCT03363191 | 1 | GoF | protect | The study was withdrawn due to an internal decision. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 13/10/2016 | https://clinicaltrials.gov/study/NCT02924688 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 15/06/2016 | https://clinicaltrials.gov/study/NCT02753712 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 4 | Completed | 13/10/2017 | https://clinicaltrials.gov/study/NCT03315000 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 22/06/2017 | https://clinicaltrials.gov/study/NCT03184987 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 09/07/2015 | https://clinicaltrials.gov/study/NCT02446418 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01498653 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT04750603 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96428df1-ea05-431a-98d3-1ec2c4b63878 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | VILANTEROL | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01706198 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | VILANTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2dbd0671-c565-40c5-bf0f-e324db26799c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | PIRBUTEROL ACETATE | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/19009s6lbl.pdf | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8ca799f1-7029-4527-9ab4-7512504bb8e2 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=30d4c387-b99c-49f8-a8bd-de23fdafb739 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=86f040d0-a073-4fa5-8997-b2fecc4a3205 | 1 | GoF | protect | |||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | Nasal congestion | DESMOPRESSIN ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d9d8442a-4722-4b41-9faa-1ee853a4cc3b | 1 | GoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | Cough | SEVOFLURANE | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01792063 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | Laryngospasm | SEVOFLURANE | targetBased | 4 | Terminated | 01/02/2008 | https://clinicaltrials.gov/study/NCT00665418 | 1 | protect | Recruitment finished earlier, because all 40 patients enrolled | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | Laryngospasm | SEVOFLURANE | targetBased | 4 | Terminated | 01/02/2008 | https://clinicaltrials.gov/study/NCT00665418 | 1 | protect | Recruitment finished earlier, because all 40 patients enrolled | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | respiratory failure | SEVOFLURANE | targetBased | 3 | Recruiting | 15/06/2020 | https://clinicaltrials.gov/study/NCT04415060 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | Cough | SEVOFLURANE | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01792063 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Active, not recruiting | 03/05/2020 | https://clinicaltrials.gov/study/NCT04235608 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 4 | Terminated | 16/04/2020 | https://clinicaltrials.gov/study/NCT04359862 | 1 | protect | Low recruitment ratio | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Suspended | 14/03/2022 | https://clinicaltrials.gov/study/NCT05259631 | 0.7 | protect | Financial problems | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 4 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02166853 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Recruiting | 24/07/2023 | https://clinicaltrials.gov/study/NCT05849779 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | respiratory failure | SEVOFLURANE | targetBased | 3 | Recruiting | 15/06/2020 | https://clinicaltrials.gov/study/NCT04415060 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Suspended | 14/03/2022 | https://clinicaltrials.gov/study/NCT05259631 | 0.7 | protect | Financial problems | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Active, not recruiting | 03/05/2020 | https://clinicaltrials.gov/study/NCT04235608 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 4 | Terminated | 16/04/2020 | https://clinicaltrials.gov/study/NCT04359862 | 1 | protect | Low recruitment ratio | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 3 | Recruiting | 24/07/2023 | https://clinicaltrials.gov/study/NCT05849779 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | acute respiratory distress syndrome | SEVOFLURANE | targetBased | 4 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02166853 | 1 | protect | ||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRROLATE | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRROLATE | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00585039 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c99a7f95-0092-48c1-9195-3431250633da | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6be0f39e-eae0-4636-b1b3-1b31fe38301c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=206bbdec-04f0-40e3-a259-32fc6bce20d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6925ab5e-4f50-4d04-b55a-8a6f74474382 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1b7537a3-409e-4d10-87db-302f05ce684e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35eb525b-72a5-4ef9-a36a-531e9e6b3560 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f3ce78d9-e923-400e-b3cd-3e6124ac7f6d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=322c7883-f01b-4ce9-b667-4924717a4e6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=24b4489f-9e6d-4895-bf8b-a6b31050b1b2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c47c47d-45f7-4eb4-b1f8-7d6c633a1f69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4be7615b-70a5-4912-b4a5-09656cab2187 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d91161c1-cae8-4d69-a37c-fb7b64b7736a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=91b2b443-bdc7-4045-bb94-906199125f49 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7e2644e6-36c5-4988-8e52-bec90e2cd2f0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4c4cf6b3-36d4-4b41-84e9-682012d7e4e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1e9d249-33d1-4b14-b92e-8f4dccded1da | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1700a5b8-4029-424a-bfed-15f6737dd210 | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE HYDROCHLORIDE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE HYDROCHLORIDE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | acute respiratory distress syndrome | NALBUPHINE HYDROCHLORIDE | targetBased | 4 | Recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06037330 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ISOETHARINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC06 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | ISOETHARINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | BITOLTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC17 | 1 | GoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Airway obstruction | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03BB06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic bronchitis | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | Bronchiectasis | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 01/09/2020 | https://clinicaltrials.gov/study/NCT04509661 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01154127 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/sialanar | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=260e843d-a627-4cb1-8cab-4f0537d1ca5e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02872090 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566604 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02181023 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01715298 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01709864 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01697696 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01922271 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01604278 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01119937 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2013 | https://clinicaltrials.gov/study/NCT01757015 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01120691 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02576626 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 08/04/2021 | https://clinicaltrials.gov/study/NCT04606901 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/08/2017 | https://clinicaltrials.gov/study/NCT02867761 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01005901 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/04/2014 | https://clinicaltrials.gov/study/NCT01837927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 24/06/2015 | https://clinicaltrials.gov/study/NCT02371629 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 26/09/2014 | https://clinicaltrials.gov/study/NCT02236611 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT01959516 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78166def-5e2f-42a6-a1f1-0253e5765632 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01712516 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | LoF | protect | Recruitment challenges |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01513460 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01202188 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01315249 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01613326 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01529632 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | LoF | protect | Continuing delays due to COVID-19 pandemic |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01699685 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00929110 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03364829 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01727141 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7579de3d-caad-4697-b429-760db3e68a23 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04206761 | 0.7 | LoF | protect | issues related to Covid-19 restrictions/shutdowns |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02953041 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Not yet recruiting | 23/12/2024 | https://clinicaltrials.gov/study/NCT05776927 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 05/02/2018 | https://clinicaltrials.gov/study/NCT03158311 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Completed | 28/04/2017 | https://clinicaltrials.gov/study/NCT03100825 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02622243 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | asthma | GLYCOPYRRONIUM | targetBased | 3 | Withdrawn | 01/03/2015 | https://clinicaltrials.gov/study/NCT02127697 | 0.7 | LoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | cystic fibrosis | ESTROGENS, CONJUGATED | targetBased | 4 | Recruiting | 02/05/2023 | https://clinicaltrials.gov/study/NCT05704036 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | cystic fibrosis | ESTROGENS, CONJUGATED | targetBased | 4 | Recruiting | 02/05/2023 | https://clinicaltrials.gov/study/NCT05704036 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | cystic fibrosis | ESTROGENS, CONJUGATED | targetBased | 4 | Recruiting | 02/05/2023 | https://clinicaltrials.gov/study/NCT05704036 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pharyngitis | TRAMADOL | targetBased | 4 | Recruiting | 10/09/2021 | https://clinicaltrials.gov/study/NCT04991493 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pharyngitis | TRAMADOL | targetBased | 4 | Recruiting | 10/09/2021 | https://clinicaltrials.gov/study/NCT04991493 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pharyngitis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Recruiting | 10/09/2021 | https://clinicaltrials.gov/study/NCT04991493 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pharyngitis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Recruiting | 10/09/2021 | https://clinicaltrials.gov/study/NCT04991493 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL | targetBased | 4 | Withdrawn | 01/03/2008 | https://clinicaltrials.gov/study/NCT00633776 | 1 | GoF | protect | Sponsor withdrew funding prior to study initiation |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00680056 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 23/01/2015 | https://clinicaltrials.gov/study/NCT02345161 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Unknown status | 13/06/2018 | https://clinicaltrials.gov/study/NCT03551197 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 27/03/2017 | https://clinicaltrials.gov/study/NCT03081156 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01204034 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Withdrawn | 04/12/2017 | https://clinicaltrials.gov/study/NCT03181880 | 1 | GoF | protect | Study stopped early as AZ have re-prioritised to focus on research to help bring existing and innovative medicines to more patients with asthma and COPD. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 05/02/2013 | https://clinicaltrials.gov/study/NCT01794780 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 31/05/2018 | https://clinicaltrials.gov/study/NCT03573817 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01415518 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 05/07/2016 | https://clinicaltrials.gov/study/NCT02796677 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00561886 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/12/2016 | https://clinicaltrials.gov/study/NCT02972476 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00383435 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00680056 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT01186653 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/03/2005 | https://clinicaltrials.gov/study/NCT00215436 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/08/2012 | https://clinicaltrials.gov/study/NCT01656005 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Unknown status | 01/05/2013 | https://clinicaltrials.gov/study/NCT01836016 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01946620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 14/12/2016 | https://clinicaltrials.gov/study/NCT03197818 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00476099 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/04/2005 | https://clinicaltrials.gov/study/NCT00206154 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00462540 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01253473 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00996697 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/04/2006 | https://clinicaltrials.gov/study/NCT00308191 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Not yet recruiting | 28/02/2026 | https://clinicaltrials.gov/study/NCT06234345 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/11/2014 | https://clinicaltrials.gov/study/NCT02268396 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 25/08/2015 | https://clinicaltrials.gov/study/NCT02533505 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01574651 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT01917331 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00972140 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Unknown status | 01/05/2015 | https://clinicaltrials.gov/study/NCT02477397 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01908140 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Recruiting | 09/12/2020 | https://clinicaltrials.gov/study/NCT04655170 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01787097 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00379028 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01462942 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00239421 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00259779 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01760304 | 1 | GoF | protect | Sponsor decided to stop the study due to expiration of blinded placebo . |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00932646 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01572792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT03028701 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00496470 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 24/01/2017 | https://clinicaltrials.gov/study/NCT03022097 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | GoF | protect | Continuing delays due to COVID-19 pandemic |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00393458 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00929851 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01488019 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Terminated | 01/09/2011 | https://clinicaltrials.gov/study/NCT01351792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT01853787 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00215449 | 0.7 | GoF | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 31/10/2014 | https://clinicaltrials.gov/study/NCT04520230 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2006 | https://clinicaltrials.gov/study/NCT00280371 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Recruiting | 21/02/2024 | https://clinicaltrials.gov/study/NCT06283966 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00542880 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01854645 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478696 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Withdrawn | 01/09/2011 | https://clinicaltrials.gov/study/NCT01377428 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/08/2016 | https://clinicaltrials.gov/study/NCT02988869 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT00796653 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 19/09/2011 | https://clinicaltrials.gov/study/NCT01437540 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/07/2011 | https://clinicaltrials.gov/study/NCT01397890 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00628862 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/12/2009 | https://clinicaltrials.gov/study/NCT01047553 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00793624 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT00489853 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01245569 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Recruiting | 07/07/2017 | https://clinicaltrials.gov/study/NCT03275116 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 10/08/2015 | https://clinicaltrials.gov/study/NCT02497001 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00139932 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 25/06/2018 | https://clinicaltrials.gov/study/NCT03478683 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01437397 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00421122 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00507234 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00383721 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2007 | https://clinicaltrials.gov/study/NCT00419744 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01070784 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00134979 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | GoF | protect | Recruitment challenges |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 27/04/2015 | https://clinicaltrials.gov/study/NCT02424344 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb24d6b0-0d69-4a3c-a7f4-a35ca4804657 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Withdrawn | 01/08/2011 | https://clinicaltrials.gov/study/NCT01393145 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Unknown status | 01/04/2015 | https://clinicaltrials.gov/study/NCT02526758 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/04/2005 | https://clinicaltrials.gov/study/NCT00206167 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01069289 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01748279 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00931385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 3 | Completed | 27/06/2014 | https://clinicaltrials.gov/study/NCT02157935 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | FORMOTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03AC13 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Airway obstruction | FORMOTEROL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R03CC15 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiectasis | FORMOTEROL | targetBased | 3 | Terminated | 29/01/2019 | https://clinicaltrials.gov/study/NCT03846570 | 0.7 | GoF | protect | Insufficient inclusion rate (due to COVID-19 pandemic) |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Bronchiectasis | FORMOTEROL | targetBased | 4 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01769898 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00479739 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00747318 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00413387 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/05/2006 | https://clinicaltrials.gov/study/NCT00326053 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2003 | https://clinicaltrials.gov/study/NCT00490243 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00383552 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00563056 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Recruiting | 01/10/2007 | https://clinicaltrials.gov/study/NCT01225913 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01450774 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/04/2015 | https://clinicaltrials.gov/study/NCT02574975 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 09/01/2012 | https://clinicaltrials.gov/study/NCT01471340 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01001364 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Withdrawn | 11/06/2020 | https://clinicaltrials.gov/study/NCT04271839 | 1 | GoF | protect | The trial has been terminated early due to the SARS-CoV-2 pandemic. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01345916 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT00252824 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00290264 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 28/05/2014 | https://clinicaltrials.gov/study/NCT02062463 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01892787 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05322707 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/11/2005 | https://clinicaltrials.gov/study/NCT00255255 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00394199 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00651547 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Terminated | 01/02/2010 | https://clinicaltrials.gov/study/NCT01070888 | 1 | GoF | protect | Unable to reach target goal |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/07/2009 | https://clinicaltrials.gov/study/NCT00914654 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Recruiting | 22/07/2022 | https://clinicaltrials.gov/study/NCT04191434 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01099722 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00734318 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/01/2009 | https://clinicaltrials.gov/study/NCT00900874 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 10/01/2019 | https://clinicaltrials.gov/study/NCT03788395 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Terminated | 23/08/2021 | https://clinicaltrials.gov/study/NCT04705727 | 0.7 | GoF | protect | Not enough recruitment in th trial |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb24d6b0-0d69-4a3c-a7f4-a35ca4804657 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT02388373 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00327353 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00319306 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/02/2007 | https://clinicaltrials.gov/study/NCT00419952 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT03015259 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00242411 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2020 | https://clinicaltrials.gov/study/NCT04215848 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00497237 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566149 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00691951 | 0.7 | GoF | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Recruiting | 01/05/2020 | https://clinicaltrials.gov/study/NCT04171180 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 04/07/2013 | https://clinicaltrials.gov/study/NCT01803555 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00649025 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/05/2004 | https://clinicaltrials.gov/study/NCT00238784 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00646321 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/05/2005 | https://clinicaltrials.gov/study/NCT00242775 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702325 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/08/2002 | https://clinicaltrials.gov/study/NCT00651651 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/05/2005 | https://clinicaltrials.gov/study/NCT00244608 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Enrolling by invitation | 01/01/2020 | https://clinicaltrials.gov/study/NCT04185129 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/09/2008 | https://clinicaltrials.gov/study/NCT00867737 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00849095 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 09/12/2005 | https://clinicaltrials.gov/study/NCT01089322 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00939341 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00460577 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01570478 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 09/07/2015 | https://clinicaltrials.gov/study/NCT02446418 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Terminated | 01/05/2013 | https://clinicaltrials.gov/study/NCT01845025 | 1 | GoF | protect | Due to the action to withdraw the Foradil Aerolizer NDA in US; study was discontinued. This was a commercial reason and not due to any change in benefit-risk. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT01255579 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/08/2004 | https://clinicaltrials.gov/study/NCT00862264 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00646009 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00393952 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00393991 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00424008 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00252863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00288379 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00652392 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00235911 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00901368 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00476073 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00383240 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Recruiting | 02/06/2017 | https://clinicaltrials.gov/study/NCT03387241 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00652002 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00259766 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01511367 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=116464ce-cfd8-4b9f-8b5a-3a114b6ff2b1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/08/2003 | https://clinicaltrials.gov/study/NCT00651768 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/01/2015 | https://clinicaltrials.gov/study/NCT02345993 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Recruiting | 22/07/2022 | https://clinicaltrials.gov/study/NCT04191447 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00272753 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/02/2004 | https://clinicaltrials.gov/study/NCT00476268 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT00130351 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01167010 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00449527 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00646516 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01202084 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Terminated | 11/11/2013 | https://clinicaltrials.gov/study/NCT01912872 | 1 | GoF | protect | Termination was due to identified errors in data management handling after an internal audit by the sponsor performed by third party. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00381485 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00394121 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00449501 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00646620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00915538 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00646529 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 15/06/2016 | https://clinicaltrials.gov/study/NCT02753712 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 09/10/2017 | https://clinicaltrials.gov/study/NCT03453112 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00252785 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 13/01/2017 | https://clinicaltrials.gov/study/NCT02495168 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 04/03/2014 | https://clinicaltrials.gov/study/NCT02045875 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT01676987 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00394368 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/09/2015 | https://clinicaltrials.gov/study/NCT02934945 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Unknown status | 01/02/2012 | https://clinicaltrials.gov/study/NCT01404013 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00463866 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00536913 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Withdrawn | 01/02/2014 | https://clinicaltrials.gov/study/NCT01658891 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT02725242 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Withdrawn | 01/06/2010 | https://clinicaltrials.gov/study/NCT00997477 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Terminated | 01/09/2006 | https://clinicaltrials.gov/study/NCT00385593 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 28/11/2014 | https://clinicaltrials.gov/study/NCT02224157 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/01/2007 | https://clinicaltrials.gov/study/NCT00419757 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00861926 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/12/2014 | https://clinicaltrials.gov/study/NCT02308098 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Recruiting | 31/01/2023 | https://clinicaltrials.gov/study/NCT05831566 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Active, not recruiting | 05/10/2022 | https://clinicaltrials.gov/study/NCT05292586 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00259792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01475032 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00475813 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/11/2011 | https://clinicaltrials.gov/study/NCT01620099 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00862394 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Terminated | 31/08/2015 | https://clinicaltrials.gov/study/NCT02491970 | 1 | GoF | protect | Difficulty of patients enrollments |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 30/03/2011 | https://clinicaltrials.gov/study/NCT01290874 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 07/07/2014 | https://clinicaltrials.gov/study/NCT02149199 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00910793 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00646594 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 3 | Unknown status | 01/03/2011 | https://clinicaltrials.gov/study/NCT01602523 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00680056 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL | targetBased | 4 | Withdrawn | 01/03/2008 | https://clinicaltrials.gov/study/NCT00633776 | 1 | GoF | protect | Sponsor withdrew funding prior to study initiation |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | FORMOTEROL | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | TERBUTALINE SULFATE | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00421122 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2a4be57a-a3df-4aa5-be52-845b75e78b3b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94742710-e87e-4e1c-85ee-0a2e7a77f0de | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8265c67f-55bc-40f0-9ede-3e4b4bb178f4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fbf54709-5857-4dbc-9233-ebd7abff88c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=382dcebb-6e41-4335-818d-b5822e3fb884 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a95bc4f-34d3-4b5e-841b-6839e1c1e0c7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=352e3476-2bc4-4995-9fd9-4a8864417f1a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=879be756-991a-42c5-a381-9c8c2ad1fbdb | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ed2af9a9-8bc4-4c6a-b8df-e8fc88c93d6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=613df3cb-4d55-4d12-ab08-a932481aaa0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7474ef3d-036f-47dd-aea7-be6d1086c0e4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4ae8fb3d-9df3-4948-bb07-6e4169521f15 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cec31032-f366-4524-9e01-63146e473b2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | TERBUTALINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0a95bc4f-34d3-4b5e-841b-6839e1c1e0c7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d06309-0483-48c6-a9f2-b25dcc31db72 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f2b67032-2ad0-4db5-a8b5-f1d3943f65d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fe2669f-ca2a-fd31-e09c-fd2819a8308c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a7c031a5-3bf8-4548-8fe7-0c308a45152a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00462540 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e95d573-74b2-4b0e-9397-c2cdaf7f41e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdda27b-e123-4f33-87e2-63f33b2b061e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05f7916c-52f7-4a4f-a19b-f5e35f02fd0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cad98bce-bb10-4a04-94e7-d6f55ae60cda | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=254422f6-37e0-4dc1-ad3b-b2e20009a7b5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=953ab65b-b157-41b3-8f90-98fa9d7f20c5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5daaf067-49a2-4067-afa4-39411c87524f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d215fe3-1cf6-4bb3-b005-75f1a9d62235 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d92c5d6b-ff10-4087-36a2-1cfc464cb967 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9832312e-0310-4ab6-8501-afa976fc7eb2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=16efdd16-04db-4874-92de-d1de22aee074 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=071e819b-43cd-48f7-b63e-c891936dcfc4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=829f381a-7888-4579-a2c5-4359764813c3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26138eb9-ff04-41cc-94c8-83edcba78b86 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f0d280b-747e-4232-9d4d-a5c964f0daad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1821db2-8a50-40e1-8448-4e59eafd2612 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=133fb50a-68d3-4d1b-a47a-67d07cc48da5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e439746-9808-4102-a92f-bf6c3f1e3513 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a56892b-7485-4570-9a1d-53ef23f07cbe | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5c2fe10-c61f-4cbe-bc45-7ab14baa691c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8e701d71-1dcb-4b84-bada-84e4f04f5e62 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bcc115b-88da-4ff0-b4e1-ef10d5287e54 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=708615b8-79b1-4eaa-b765-abb01be3233a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b2ff7e6-d9fb-47ed-85eb-859cb1bc8a79 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=77f24a8c-0a9a-4708-a401-3f56b5d4f2c1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ecc00500-58b8-491f-80df-dc6c89a08cae | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab1cbed4-50c4-4769-9947-1927333f278c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=938ab43d-bf4c-477f-b918-aabe738d562b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=374cbcbb-a37c-4057-b9a9-f70b3fdb803c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f8839cf1-ec1c-42e6-a4d3-b089e95337e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=285a656c-f77f-4e77-b0a8-a0e9c901803f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=34b6ab14-9ed5-4651-9be6-84685e6aa919 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b49edc01-1ea6-4f88-883a-bb4bc2bf4b3f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a9d01507-ae72-4322-93d5-5d857b1b2caa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b72742df-1a6b-4483-aff3-3e79db5e015d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e65e8c22-d9d9-4473-8240-cfd154b8e6c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ec64742-5802-49f0-bbbe-95580730c0a7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58860ee1-5a9d-4422-b9bc-78e8b7958b53 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fbbb275-24a8-43c6-b341-baf90957cd69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e7c1e97-b8b2-44c0-aeac-18f9142a522b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3c64255e-074c-41f8-a344-3686a0685020 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0d31515-28cf-4041-9007-a5a9b52dc78d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a90911e-adb5-4331-8d1a-f791fba549f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/02/2002 | https://clinicaltrials.gov/study/NCT00665600 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 3 | Completed | 28/09/2017 | https://clinicaltrials.gov/study/NCT03256695 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=774b1ef9-015e-4e8e-9c52-d305de1ef85e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | Completed | 02/12/2020 | https://clinicaltrials.gov/study/NCT04606394 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f422539-5708-46fd-944d-c88a12fc5ec3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=574824f3-51cc-4b94-9c10-e3204d8b19f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53176c67-ebcd-4b07-a08a-f2df3c541af9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=acc9a53e-304d-44e4-b4ab-5303d1361118 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c53c391b-de2f-4df8-8c25-6764564580c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1c1f3881-2b68-4bdb-a12f-8134fe8ba961 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | atopic asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 09/05/2018 | https://clinicaltrials.gov/study/NCT03468790 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=87b8cd3c-2849-4b50-b63e-9ea379165c07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29f0d08e-14d7-4659-bc00-0d84ae69cb7f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3d2ab1e-8f91-4a4d-aa6b-5a57f59ad56b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0ec9932-b2a3-4776-a7e8-2aeded019da4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74170607-50c0-4de5-847e-64da9b1d52db | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=69ffacaa-c7e5-4016-9e77-79f7d724baad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ffb3297-6d69-4475-9bb3-ccecf3ede614 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0dee492-8bf9-4360-be9b-9d6ee1ecb256 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f454947c-6055-474b-bf35-29a379e01aa3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6dc143e7-6bab-42c8-b3e9-9d604d202e73 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3544356-e36a-4ce6-8235-cb0531c658ad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1bb952b7-8826-40a1-ac7b-38ec1bd9b94e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=afcf38bf-b183-4569-b43f-25deb97f6693 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cb4f1002-9372-4fac-aea3-99142f93dd37 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d8f090fb-c591-4d6a-98b7-925b0bb17c38 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42bfd443-1d9d-4806-b044-6ff874b92b25 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a801d6-9573-4997-9b4a-aec6db6459ed | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=911f605d-5451-49e2-a484-00f619f2012b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=23c05e9a-9df5-4978-8831-cf562aa4ba92 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a9b20e4-82e6-4151-9c9d-39aaf2a767e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a1cb095-c3c6-4629-9a88-aeae949541ef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021527s021lbl.pdf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99d6ba1a-1599-48a3-8dbd-a8b06af20fe1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=22367ff1-5edf-429a-955a-728e42695f60 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5329614f-ae0b-4854-bb6f-ea0b7dae87c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cbcc9dbb-5758-4979-b84b-891f3d04f61e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae35ec1f-526a-49bf-8262-c50e40190d1f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f248b57a-2c06-4ed8-817e-e6ff42f5ce88 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ecc00500-58b8-491f-80df-dc6c89a08cae | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a1cb095-c3c6-4629-9a88-aeae949541ef | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e439746-9808-4102-a92f-bf6c3f1e3513 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00646620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6b2ff7e6-d9fb-47ed-85eb-859cb1bc8a79 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=acc9a53e-304d-44e4-b4ab-5303d1361118 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=05f7916c-52f7-4a4f-a19b-f5e35f02fd0b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | Active, not recruiting | 09/07/2021 | https://clinicaltrials.gov/study/NCT04997304 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b49edc01-1ea6-4f88-883a-bb4bc2bf4b3f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a56892b-7485-4570-9a1d-53ef23f07cbe | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=071e819b-43cd-48f7-b63e-c891936dcfc4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a7c031a5-3bf8-4548-8fe7-0c308a45152a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a90911e-adb5-4331-8d1a-f791fba549f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/04/2016 | https://clinicaltrials.gov/study/NCT02584257 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00577655 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=16efdd16-04db-4874-92de-d1de22aee074 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=22367ff1-5edf-429a-955a-728e42695f60 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1bb952b7-8826-40a1-ac7b-38ec1bd9b94e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00144846 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1c1f3881-2b68-4bdb-a12f-8134fe8ba961 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3c64255e-074c-41f8-a344-3686a0685020 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5daaf067-49a2-4067-afa4-39411c87524f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=574824f3-51cc-4b94-9c10-e3204d8b19f8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=911f605d-5451-49e2-a484-00f619f2012b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7bdda27b-e123-4f33-87e2-63f33b2b061e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02126839 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=374cbcbb-a37c-4057-b9a9-f70b3fdb803c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=34b6ab14-9ed5-4651-9be6-84685e6aa919 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5329614f-ae0b-4854-bb6f-ea0b7dae87c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ffb3297-6d69-4475-9bb3-ccecf3ede614 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Not yet recruiting | 11/12/2023 | https://clinicaltrials.gov/study/NCT06154304 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=77f24a8c-0a9a-4708-a401-3f56b5d4f2c1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fa5ebedb-069c-4a1e-9a25-b6fa957e6c2b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1821db2-8a50-40e1-8448-4e59eafd2612 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=953ab65b-b157-41b3-8f90-98fa9d7f20c5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8e701d71-1dcb-4b84-bada-84e4f04f5e62 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e7c1e97-b8b2-44c0-aeac-18f9142a522b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f454947c-6055-474b-bf35-29a379e01aa3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a9d01507-ae72-4322-93d5-5d857b1b2caa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae35ec1f-526a-49bf-8262-c50e40190d1f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53176c67-ebcd-4b07-a08a-f2df3c541af9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00861926 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 22/09/2018 | https://clinicaltrials.gov/study/NCT03528577 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=becf2107-bbc9-4b7b-87a5-3221ff6fc5cf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6afb1ec5-6771-4ce7-accb-fdff05ebf3b9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b72742df-1a6b-4483-aff3-3e79db5e015d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=23c05e9a-9df5-4978-8831-cf562aa4ba92 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cad98bce-bb10-4a04-94e7-d6f55ae60cda | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fbbb275-24a8-43c6-b341-baf90957cd69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cbcc9dbb-5758-4979-b84b-891f3d04f61e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9832312e-0310-4ab6-8501-afa976fc7eb2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4f0d280b-747e-4232-9d4d-a5c964f0daad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f2b67032-2ad0-4db5-a8b5-f1d3943f65d8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=285a656c-f77f-4e77-b0a8-a0e9c901803f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e65e8c22-d9d9-4473-8240-cfd154b8e6c8 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Withdrawn | 01/04/2022 | https://clinicaltrials.gov/study/NCT05292976 | 0.7 | GoF | protect | Business reasons |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=774b1ef9-015e-4e8e-9c52-d305de1ef85e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 20/03/2019 | https://clinicaltrials.gov/study/NCT03847896 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | Completed | 27/07/2020 | https://clinicaltrials.gov/study/NCT04159519 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=69ffacaa-c7e5-4016-9e77-79f7d724baad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=133fb50a-68d3-4d1b-a47a-67d07cc48da5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=58860ee1-5a9d-4422-b9bc-78e8b7958b53 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=708615b8-79b1-4eaa-b765-abb01be3233a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e95d573-74b2-4b0e-9397-c2cdaf7f41e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26138eb9-ff04-41cc-94c8-83edcba78b86 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=938ab43d-bf4c-477f-b918-aabe738d562b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f248b57a-2c06-4ed8-817e-e6ff42f5ce88 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Terminated | 01/02/2008 | https://clinicaltrials.gov/study/NCT00634829 | 0.7 | GoF | protect | IND voluntarily withdrawn, without prejudice |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3d2ab1e-8f91-4a4d-aa6b-5a57f59ad56b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | Completed | 01/05/1998 | https://clinicaltrials.gov/study/NCT02182713 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12d06309-0483-48c6-a9f2-b25dcc31db72 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00634517 | 0.7 | GoF | protect | IND voluntarily withdrawn, without prejudice |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d215fe3-1cf6-4bb3-b005-75f1a9d62235 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6dc143e7-6bab-42c8-b3e9-9d604d202e73 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 13/02/2017 | https://clinicaltrials.gov/study/NCT02969408 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=87b8cd3c-2849-4b50-b63e-9ea379165c07 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74170607-50c0-4de5-847e-64da9b1d52db | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205636s004lbl.pdf | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42bfd443-1d9d-4806-b044-6ff874b92b25 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0dee492-8bf9-4360-be9b-9d6ee1ecb256 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7fe2669f-ca2a-fd31-e09c-fd2819a8308c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a801d6-9573-4997-9b4a-aec6db6459ed | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99d6ba1a-1599-48a3-8dbd-a8b06af20fe1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=829f381a-7888-4579-a2c5-4359764813c3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=254422f6-37e0-4dc1-ad3b-b2e20009a7b5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0ec9932-b2a3-4776-a7e8-2aeded019da4 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bcc115b-88da-4ff0-b4e1-ef10d5287e54 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e4a92e7c-6d19-45e7-91f0-685f68383784 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | Completed | 09/12/2019 | https://clinicaltrials.gov/study/NCT04207840 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d3544356-e36a-4ce6-8235-cb0531c658ad | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cb4f1002-9372-4fac-aea3-99142f93dd37 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c53c391b-de2f-4df8-8c25-6764564580c0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5c2fe10-c61f-4cbe-bc45-7ab14baa691c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d8f090fb-c591-4d6a-98b7-925b0bb17c38 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6f422539-5708-46fd-944d-c88a12fc5ec3 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab1cbed4-50c4-4769-9947-1927333f278c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 13/12/2018 | https://clinicaltrials.gov/study/NCT03769090 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0d31515-28cf-4041-9007-a5a9b52dc78d | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=29f0d08e-14d7-4659-bc00-0d84ae69cb7f | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f8839cf1-ec1c-42e6-a4d3-b089e95337e5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00394329 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8a9b20e4-82e6-4151-9c9d-39aaf2a767e2 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=afcf38bf-b183-4569-b43f-25deb97f6693 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Recruiting | 30/03/2023 | https://clinicaltrials.gov/study/NCT05884970 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | Completed | 11/11/2021 | https://clinicaltrials.gov/study/NCT05084222 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d92c5d6b-ff10-4087-36a2-1cfc464cb967 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2ec64742-5802-49f0-bbbe-95580730c0a7 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | ALBUTEROL SULFATE | targetBased | 3 | Completed | 01/11/2000 | https://clinicaltrials.gov/study/NCT00667407 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=73b55902-273a-4734-8deb-af6eb68b5523 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=46633af4-98c1-471f-9232-d719c6d8e445 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e4a4021c-c092-41bf-91ed-ef483fd52732 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | METAPROTERENOL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=43ff5156-e08b-4dc4-93a6-8c727676daa1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | METAPROTERENOL SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cf566e38-f6ff-4b86-9537-8684943b36eb | 1 | GoF | protect | |||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | asthma | ALFACALCIDOL | targetBased | 4 | Completed | 01/08/2014 | https://clinicaltrials.gov/study/NCT02747381 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | INDACATEROL MALEATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL MALEATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL MALEATE | targetBased | 4 | Completed | 27/08/2014 | https://clinicaltrials.gov/study/NCT02202616 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL MALEATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/onbrez-breezhaler | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL MALEATE | targetBased | 4 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01437748 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | INDACATEROL MALEATE | targetBased | 4 | Completed | 14/02/2014 | https://clinicaltrials.gov/study/NCT01985334 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | INDACATEROL MALEATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f7d013b1-5ee9-4126-8297-9efd9a5a8344 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL TARTRATE | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | ARFORMOTEROL TARTRATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 4 | Terminated | 01/11/2014 | https://clinicaltrials.gov/study/NCT02275481 | 1 | GoF | protect | lack of enrollment |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 3 | Completed | 01/02/2002 | https://clinicaltrials.gov/study/NCT00685841 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00571428 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 4 | Terminated | 03/10/2017 | https://clinicaltrials.gov/study/NCT03219866 | 1 | GoF | protect | Lower enrollment than Sponsor expected - Sponsor stopped study |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00754546 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | ARFORMOTEROL TARTRATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL TARTRATE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL TARTRATE | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00064402 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | ARFORMOTEROL TARTRATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7134ae7c-6c64-470d-ab4e-81e35413b839 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bronchitis | ARFORMOTEROL TARTRATE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT00250679 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | chronic bronchitis | IVACAFTOR | targetBased | 4 | Withdrawn | 01/01/2020 | https://clinicaltrials.gov/study/NCT03251911 | 1 | GoF | protect | no participants enrolled |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | chronic bronchitis | IVACAFTOR | targetBased | 4 | Withdrawn | 01/01/2020 | https://clinicaltrials.gov/study/NCT03251911 | 1 | GoF | protect | no participants enrolled |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | respiratory system disease | IVACAFTOR | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R07AX02 | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | respiratory system disease | IVACAFTOR | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=R07AX02 | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01117012 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01117012 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01705145 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01705145 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/01/2015 | https://clinicaltrials.gov/study/NCT02347657 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/01/2015 | https://clinicaltrials.gov/study/NCT02347657 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Terminated | 01/10/2018 | https://clinicaltrials.gov/study/NCT03956589 | 0.5 | GoF | protect | Patient inclusion has stopped after interim analysis. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Terminated | 01/10/2018 | https://clinicaltrials.gov/study/NCT03956589 | 0.5 | GoF | protect | Patient inclusion has stopped after interim analysis. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01807923 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01807923 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Unknown status | 01/02/2014 | https://clinicaltrials.gov/study/NCT01937325 | 1 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Unknown status | 01/02/2014 | https://clinicaltrials.gov/study/NCT01937325 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01807949 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01807949 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 16/08/2017 | https://clinicaltrials.gov/study/NCT03277196 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 16/08/2017 | https://clinicaltrials.gov/study/NCT03277196 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 24/02/2020 | https://clinicaltrials.gov/study/NCT04235140 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 24/02/2020 | https://clinicaltrials.gov/study/NCT04235140 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 17/05/2018 | https://clinicaltrials.gov/study/NCT03559062 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 17/05/2018 | https://clinicaltrials.gov/study/NCT03559062 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f354423a-85c2-41c3-a9db-0f3aee135d8d | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f354423a-85c2-41c3-a9db-0f3aee135d8d | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 02/06/2016 | https://clinicaltrials.gov/study/NCT02725567 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 02/06/2016 | https://clinicaltrials.gov/study/NCT02725567 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01614470 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01614470 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 28/08/2019 | https://clinicaltrials.gov/study/NCT04058353 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 28/08/2019 | https://clinicaltrials.gov/study/NCT04058353 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 18/07/2017 | https://clinicaltrials.gov/study/NCT03068312 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 18/07/2017 | https://clinicaltrials.gov/study/NCT03068312 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/06/2015 | https://clinicaltrials.gov/study/NCT02412111 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/06/2015 | https://clinicaltrials.gov/study/NCT02412111 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02953314 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02953314 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01614457 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01614457 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3fc1c40e-cfac-47a1-9e1a-61ead3570600 | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3fc1c40e-cfac-47a1-9e1a-61ead3570600 | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02392234 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02392234 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/12/2013 | https://clinicaltrials.gov/study/NCT01946412 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/12/2013 | https://clinicaltrials.gov/study/NCT01946412 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=302ae804-37db-44fd-ac2f-3dbdeda9aa4b | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=302ae804-37db-44fd-ac2f-3dbdeda9aa4b | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02544451 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02544451 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 07/09/2018 | https://clinicaltrials.gov/study/NCT03601637 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 07/09/2018 | https://clinicaltrials.gov/study/NCT03601637 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/kalydeco | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02565914 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02565914 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Recruiting | 21/06/2022 | https://clinicaltrials.gov/study/NCT05422222 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Recruiting | 21/06/2022 | https://clinicaltrials.gov/study/NCT05422222 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02516410 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02516410 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00909727 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00909727 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 08/11/2022 | https://clinicaltrials.gov/study/NCT05444257 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Active, not recruiting | 08/11/2022 | https://clinicaltrials.gov/study/NCT05444257 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 25/04/2018 | https://clinicaltrials.gov/study/NCT03537651 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 25/04/2018 | https://clinicaltrials.gov/study/NCT03537651 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01707290 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT01707290 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 19/06/2020 | https://clinicaltrials.gov/study/NCT04353817 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 19/06/2020 | https://clinicaltrials.gov/study/NCT04353817 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02514473 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02514473 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02934698 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02934698 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0ab0c9f8-3eee-4e0f-9f3f-c1e16aaffe25 | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0ab0c9f8-3eee-4e0f-9f3f-c1e16aaffe25 | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01931839 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01931839 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02742519 | 0.7 | GoF | protect | Low enrollment. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02742519 | 0.7 | GoF | protect | Low enrollment. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 03/10/2019 | https://clinicaltrials.gov/study/NCT04105972 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 03/10/2019 | https://clinicaltrials.gov/study/NCT04105972 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 12/05/2017 | https://clinicaltrials.gov/study/NCT03125395 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 12/05/2017 | https://clinicaltrials.gov/study/NCT03125395 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01897233 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01897233 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 24/05/2017 | https://clinicaltrials.gov/study/NCT03150719 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 24/05/2017 | https://clinicaltrials.gov/study/NCT03150719 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00909532 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | IVACAFTOR | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00909532 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | MORPHINE SULFATE | targetBased | 4 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01236495 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | lung disease | MORPHINE SULFATE | targetBased | 4 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01236495 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02514473 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02514473 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 4 | Terminated | 01/10/2018 | https://clinicaltrials.gov/study/NCT03956589 | 0.5 | GoF | protect | Patient inclusion has stopped after interim analysis. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 4 | Terminated | 01/10/2018 | https://clinicaltrials.gov/study/NCT03956589 | 0.5 | GoF | protect | Patient inclusion has stopped after interim analysis. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01807949 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01807949 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3fc1c40e-cfac-47a1-9e1a-61ead3570600 | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3fc1c40e-cfac-47a1-9e1a-61ead3570600 | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01897233 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01897233 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01807923 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01807923 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02544451 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02544451 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 24/02/2020 | https://clinicaltrials.gov/study/NCT04235140 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 24/02/2020 | https://clinicaltrials.gov/study/NCT04235140 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 12/05/2017 | https://clinicaltrials.gov/study/NCT03125395 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 12/05/2017 | https://clinicaltrials.gov/study/NCT03125395 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01931839 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | LUMACAFTOR | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01931839 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | OLODATEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01e15aee-40e0-23f3-537f-c96dd63e2cb1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | OLODATEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | OLODATEROL HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d9a5433-857e-44ea-b3d6-2a400ca0cef5 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Nasal congestion | EPHEDRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1239e824-aa60-4b7f-9910-81f96ebe6c70 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=750f9a39-acdc-4abc-800e-467884a4dcea | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=172e970d-6773-4796-a280-3370e2f46c69 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | EPHEDRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2170f081-c03f-47e8-bc63-4052c5e653fa | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | common cold | EPHEDRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1239e824-aa60-4b7f-9910-81f96ebe6c70 | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf | 1 | GoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210491lbl.pdf | 1 | GoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02565914 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02565914 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 25/04/2018 | https://clinicaltrials.gov/study/NCT03537651 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 25/04/2018 | https://clinicaltrials.gov/study/NCT03537651 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 19/06/2020 | https://clinicaltrials.gov/study/NCT04353817 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 19/06/2020 | https://clinicaltrials.gov/study/NCT04353817 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 24/05/2017 | https://clinicaltrials.gov/study/NCT03150719 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 24/05/2017 | https://clinicaltrials.gov/study/NCT03150719 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/06/2015 | https://clinicaltrials.gov/study/NCT02412111 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/06/2015 | https://clinicaltrials.gov/study/NCT02412111 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02392234 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02392234 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02953314 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02953314 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Recruiting | 21/06/2022 | https://clinicaltrials.gov/study/NCT05422222 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Recruiting | 21/06/2022 | https://clinicaltrials.gov/study/NCT05422222 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/01/2015 | https://clinicaltrials.gov/study/NCT02347657 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/01/2015 | https://clinicaltrials.gov/study/NCT02347657 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 28/08/2019 | https://clinicaltrials.gov/study/NCT04058353 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 28/08/2019 | https://clinicaltrials.gov/study/NCT04058353 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 03/10/2019 | https://clinicaltrials.gov/study/NCT04105972 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 03/10/2019 | https://clinicaltrials.gov/study/NCT04105972 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 17/05/2018 | https://clinicaltrials.gov/study/NCT03559062 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 17/05/2018 | https://clinicaltrials.gov/study/NCT03559062 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02516410 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02516410 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 08/11/2022 | https://clinicaltrials.gov/study/NCT05444257 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Active, not recruiting | 08/11/2022 | https://clinicaltrials.gov/study/NCT05444257 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | TEZACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | pulmonary arterial hypertension | AV-101 | targetBased | 3 | Active, not recruiting | 02/11/2022 | https://clinicaltrials.gov/study/NCT05557942 | 0.7 | LoF | protect | ||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | chronic obstructive pulmonary disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | lung disease | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=954859c0-a121-34d6-e053-2a95a90a1e19 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | Apnea | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00389909 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | lung disease | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=954859c0-a121-34d6-e053-2a95a90a1e19 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | hypoxia | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02171910 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | Apnea | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00389909 | 1 | LoF | protect | |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | hypoxia | DOXAPRAM HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2016 | https://clinicaltrials.gov/study/NCT02171910 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL TARTRATE | targetBased | 3 | Completed | 01/05/2002 | https://clinicaltrials.gov/study/NCT00073827 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL TARTRATE | targetBased | 3 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT00064389 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL TARTRATE | targetBased | 3 | Terminated | 01/07/2014 | https://clinicaltrials.gov/study/NCT02150499 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | LEVALBUTEROL TARTRATE | targetBased | 3 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00073814 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL FUMARATE | targetBased | 4 | Withdrawn | 01/03/2008 | https://clinicaltrials.gov/study/NCT00633776 | 1 | GoF | protect | Sponsor withdrew funding prior to study initiation |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic bronchitis | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 25/05/2021 | https://clinicaltrials.gov/study/NCT04876677 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01462942 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2005 | https://clinicaltrials.gov/study/NCT00215436 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00932646 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01488019 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Recruiting | 24/10/2023 | https://clinicaltrials.gov/study/NCT06067828 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT01853787 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Not yet recruiting | 15/02/2021 | https://clinicaltrials.gov/study/NCT04737655 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2006 | https://clinicaltrials.gov/study/NCT00308191 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb24d6b0-0d69-4a3c-a7f4-a35ca4804657 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Withdrawn | 04/10/2021 | https://clinicaltrials.gov/study/NCT04671355 | 1 | GoF | protect | Continuing delays due to COVID-19 pandemic |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Recruiting | 07/07/2017 | https://clinicaltrials.gov/study/NCT03275116 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00139932 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 05/07/2016 | https://clinicaltrials.gov/study/NCT02796677 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00507234 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Withdrawn | 04/12/2017 | https://clinicaltrials.gov/study/NCT03181880 | 1 | GoF | protect | Study stopped early as AZ have re-prioritised to focus on research to help bring existing and innovative medicines to more patients with asthma and COPD. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Recruiting | 29/02/2024 | https://clinicaltrials.gov/study/NCT06282861 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/05/2015 | https://clinicaltrials.gov/study/NCT02467452 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 10/08/2015 | https://clinicaltrials.gov/study/NCT02497001 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 09/08/2016 | https://clinicaltrials.gov/study/NCT03262012 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/11/2014 | https://clinicaltrials.gov/study/NCT02268396 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00931385 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 25/08/2015 | https://clinicaltrials.gov/study/NCT02533505 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/11/2013 | https://clinicaltrials.gov/study/NCT01970878 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01911364 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 14/12/2016 | https://clinicaltrials.gov/study/NCT03197818 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01946620 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Withdrawn | 19/01/2024 | https://clinicaltrials.gov/study/NCT05970263 | 1 | GoF | protect | Recruitment challenges |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01854658 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01245569 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 30/03/2015 | https://clinicaltrials.gov/study/NCT02343458 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01908140 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01437397 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Recruiting | 28/04/2022 | https://clinicaltrials.gov/study/NCT04320342 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01204034 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01572792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Recruiting | 21/02/2024 | https://clinicaltrials.gov/study/NCT06283966 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 24/01/2017 | https://clinicaltrials.gov/study/NCT03022097 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/12/2016 | https://clinicaltrials.gov/study/NCT02972476 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Terminated | 01/09/2011 | https://clinicaltrials.gov/study/NCT01351792 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT02937584 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 20/11/2017 | https://clinicaltrials.gov/study/NCT03268226 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00215449 | 0.7 | GoF | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 25/05/2017 | https://clinicaltrials.gov/study/NCT03162055 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 19/09/2011 | https://clinicaltrials.gov/study/NCT01437540 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01854645 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/01/2006 | https://clinicaltrials.gov/study/NCT00280371 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | chronic obstructive pulmonary disease | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00929851 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01475032 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2020 | https://clinicaltrials.gov/study/NCT04215848 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05322707 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00383552 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00413387 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Unknown status | 01/02/2012 | https://clinicaltrials.gov/study/NCT01404013 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Recruiting | 31/01/2023 | https://clinicaltrials.gov/study/NCT05831566 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 28/05/2014 | https://clinicaltrials.gov/study/NCT02062463 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=116464ce-cfd8-4b9f-8b5a-3a114b6ff2b1 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01345916 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Withdrawn | 01/02/2014 | https://clinicaltrials.gov/study/NCT01658891 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00915538 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01001364 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00475813 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Unknown status | 01/09/2008 | https://clinicaltrials.gov/study/NCT00867737 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01566149 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT01450774 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702325 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb24d6b0-0d69-4a3c-a7f4-a35ca4804657 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 13/01/2017 | https://clinicaltrials.gov/study/NCT02495168 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 10/01/2019 | https://clinicaltrials.gov/study/NCT03788395 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Enrolling by invitation | 01/01/2020 | https://clinicaltrials.gov/study/NCT04185129 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 09/12/2005 | https://clinicaltrials.gov/study/NCT01089322 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Active, not recruiting | 05/10/2022 | https://clinicaltrials.gov/study/NCT05292586 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00862394 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae02436e-9528-490e-a844-0c0f6f2843f9 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00910793 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01167010 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00252863 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00476073 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00394199 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00649025 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00393952 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00747318 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00563056 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00901368 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01570478 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00394368 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 29/12/2016 | https://clinicaltrials.gov/study/NCT03015259 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01511367 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=715433dc-cb2b-43ea-b6df-c5b4177adc6c | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 09/07/2015 | https://clinicaltrials.gov/study/NCT02446418 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01202084 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 01/11/2011 | https://clinicaltrials.gov/study/NCT01620099 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00383240 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00393991 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 09/10/2017 | https://clinicaltrials.gov/study/NCT03453112 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01099722 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 4 | Completed | 04/03/2014 | https://clinicaltrials.gov/study/NCT02045875 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00394121 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | asthma | FORMOTEROL FUMARATE | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00734318 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04212000-ec03-42a3-8b9e-3a28237f415e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fafa4cf1-99c2-43d5-73ad-51f256de3be0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL FUMARATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb2fe258-fe2e-47f6-8adf-ca75bf6f90af | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | emphysema | FORMOTEROL FUMARATE | targetBased | 4 | Withdrawn | 01/03/2008 | https://clinicaltrials.gov/study/NCT00633776 | 1 | GoF | protect | Sponsor withdrew funding prior to study initiation |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | chronic obstructive pulmonary disease | LIRAGLUTIDE | targetBased | 4 | Completed | 01/01/2018 | https://clinicaltrials.gov/study/NCT03466021 | 1 | GoF | protect | |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | chronic obstructive pulmonary disease | LIRAGLUTIDE | targetBased | 4 | Completed | 01/01/2018 | https://clinicaltrials.gov/study/NCT03466021 | 1 | GoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | chronic obstructive pulmonary disease | LIRAGLUTIDE | targetBased | 4 | Completed | 01/01/2018 | https://clinicaltrials.gov/study/NCT03466021 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Completed | 07/03/2018 | https://clinicaltrials.gov/study/NCT03447249 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Terminated | 03/08/2018 | https://clinicaltrials.gov/study/NCT03633526 | 0.7 | GoF | protect | Study discontinued after Part A due to Sponsor's discretion. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Terminated | 13/07/2018 | https://clinicaltrials.gov/study/NCT03447262 | 0.7 | GoF | protect | At Sponsor's Discretion |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | BAMOCAFTOR | targetBased | 3 | Completed | 01/05/2018 | https://clinicaltrials.gov/study/NCT03460990 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Not yet recruiting | 01/10/2024 | https://clinicaltrials.gov/study/NCT06460506 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/08/2019 | https://clinicaltrials.gov/study/NCT04043806 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 27/10/2021 | https://clinicaltrials.gov/study/NCT05076149 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 19/11/2020 | https://clinicaltrials.gov/study/NCT04537793 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Active, not recruiting | 17/01/2022 | https://clinicaltrials.gov/study/NCT05153317 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 14/01/2022 | https://clinicaltrials.gov/study/NCT05111145 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 05/12/2019 | https://clinicaltrials.gov/study/NCT04058366 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 03/08/2018 | https://clinicaltrials.gov/study/NCT03525548 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/10/2018 | https://clinicaltrials.gov/study/NCT03525574 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 09/05/2022 | https://clinicaltrials.gov/study/NCT05274269 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Recruiting | 27/06/2023 | https://clinicaltrials.gov/study/NCT05882357 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03691779 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 4 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT03624101 | 1 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 15/06/2018 | https://clinicaltrials.gov/study/NCT03525444 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 04/05/2020 | https://clinicaltrials.gov/study/NCT04362761 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04599465 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Active, not recruiting | 23/11/2022 | https://clinicaltrials.gov/study/NCT05331183 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 11/01/2021 | https://clinicaltrials.gov/study/NCT04545515 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 17/02/2020 | https://clinicaltrials.gov/study/NCT04183790 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Enrolling by invitation | 11/08/2023 | https://clinicaltrials.gov/study/NCT05844449 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 14/09/2021 | https://clinicaltrials.gov/study/NCT05033080 | 0.7 | GoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | cystic fibrosis | ELEXACAFTOR | targetBased | 3 | Completed | 12/10/2021 | https://clinicaltrials.gov/study/NCT04969224 | 0.7 | GoF | protect | |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | IL1RL1 | Interleukin-1 receptor-like 1 | chronic obstructive pulmonary disease | ASTEGOLIMAB | targetBased | 3 | Recruiting | 28/06/2023 | https://clinicaltrials.gov/study/NCT05878769 | 0.7 | LoF | protect | |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | IL1RL1 | Interleukin-1 receptor-like 1 | chronic obstructive pulmonary disease | ASTEGOLIMAB | targetBased | 3 | Recruiting | 29/12/2022 | https://clinicaltrials.gov/study/NCT05595642 | 0.7 | LoF | protect |