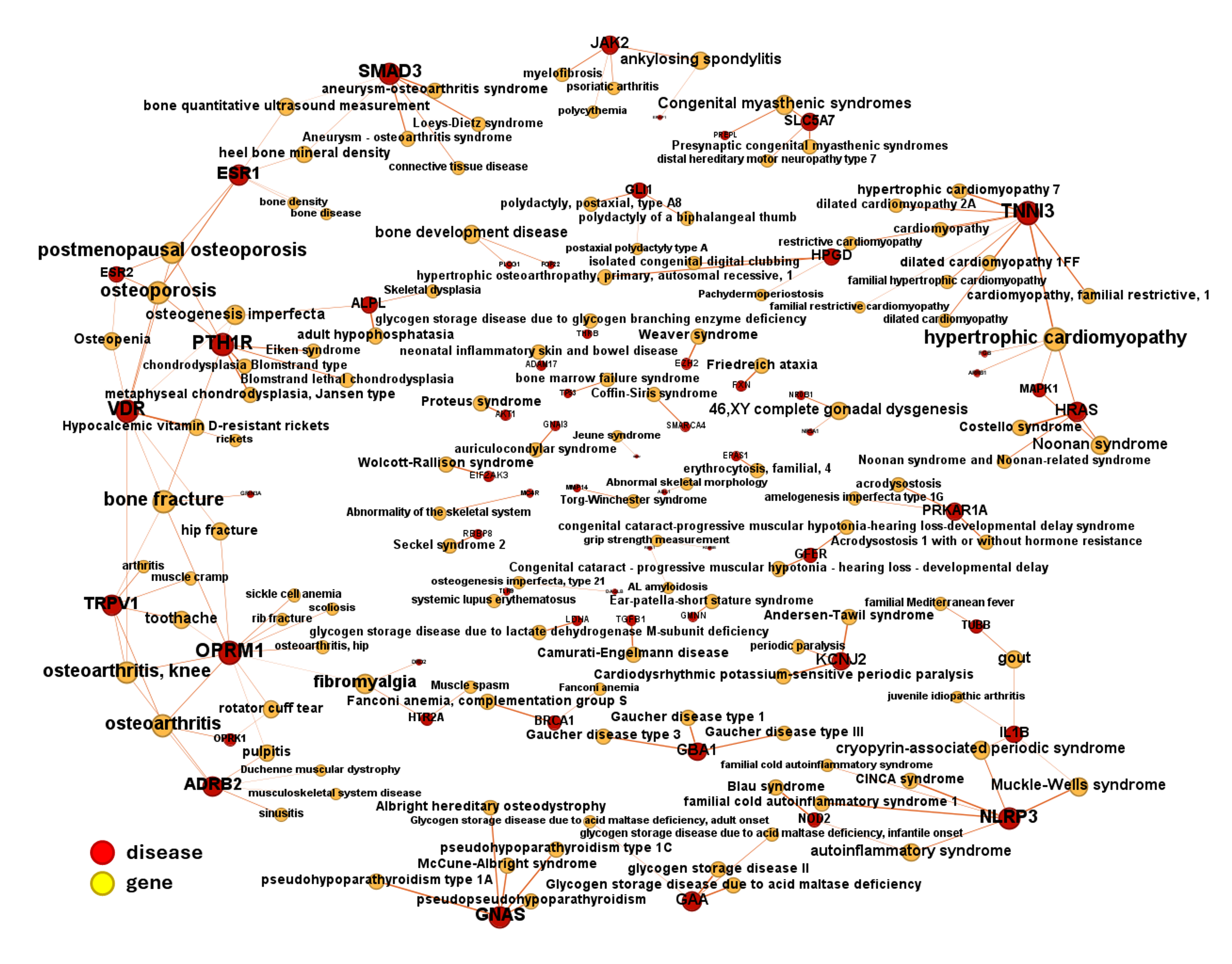
DrTarget’s AI-Driven Insights for Musculoskeletal & Connective Tissue Diseases
DrTarget leverages advanced AI and machine learning to uncover target-disease associations for musculoskeletal and connective tissue diseases, integrating data from Open Targets and other public repositories. Our computational models identify novel therapeutic targets and drug repurposing opportunities for conditions such as:
✔ Osteoarthritis & Rheumatoid Arthritis
✔ Osteoporosis & Bone Metabolic Disorders
✔ Muscular Dystrophies & Myopathies
✔ Fibrosis & Connective Tissue Disorders
✔ Joint Degeneration & Cartilage Repair
By combining phenotypic modeling and AI-driven drug discovery, we accelerate the identification of mechanisms driving disease progression, supporting the development of targeted therapies and precision medicine approaches.
Check best scored target-disease associations in table:
| BioAssay Name | program | diseaseName | assayType | testedCompounds | activeCompounds | associationScore | numberOfEvidences |
|---|---|---|---|---|---|---|---|
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | cardiomyopathy | targetBased | 335239 | 801 | 0.738160697713462 | 1141 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | cardiomyopathy | targetBased | 335238 | 390 | 0.738160697713462 | 1141 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy | targetBased | 335239 | 801 | 0.696018079292442 | 92 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy | targetBased | 335238 | 390 | 0.696018079292442 | 92 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | hypertrophic cardiomyopathy | pathwayBased | 70898 | 707 | 0.638631820518866 | 20 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | hypertrophic cardiomyopathy | pathwayBased | 131324 | 544 | 0.638631820518866 | 20 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | hypertrophic cardiomyopathy | targetBased | 335239 | 801 | 0.926253064265713 | 1241 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | hypertrophic cardiomyopathy | targetBased | 335238 | 390 | 0.926253064265713 | 1241 |
| Assay for Inhibitors of the beta-Arrestin-Adaptor Protein 2 Interaction That Mediate GPCR Degradation and Recycling | ARRB1_inhibitors | hypertrophic cardiomyopathy | targetBased | 338373 | 1061 | 0.599404989335178 | 27 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn6) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | hypertrophic cardiomyopathy | targetBased | 369953 | 760 | 0.578848118498312 | 10 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn7) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | hypertrophic cardiomyopathy | targetBased | 369953 | 498 | 0.578848118498312 | 10 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | hypertrophic cardiomyopathy | targetBased | 194628 | 267 | 0.723494176407128 | 465 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | juvenile idiopathic arthritis | pathwayBased | 362051 | 17187 | 0.557333022236428 | 217 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | psoriatic arthritis | targetBased | 217959 | 2390 | 0.629378658421174 | 63 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | restrictive cardiomyopathy | targetBased | 335239 | 801 | 0.672321932673797 | 120 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | restrictive cardiomyopathy | targetBased | 335238 | 390 | 0.672321932673797 | 120 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | osteoporosis | targetBased | 86095 | 1442 | 0.740989460833532 | 72 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | osteoporosis | targetBased | 86095 | 1151 | 0.740989460833532 | 72 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | osteoporosis | targetBased | 394050 | 3624 | 0.683681888899659 | 113 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | osteoporosis | targetBased | 86095 | 1114 | 0.661909666301374 | 61 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | osteoporosis | targetBased | 405685 | 308 | 0.658783124032757 | 133 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | cutaneous lupus erythematosus | targetBased | 343468 | 734 | 0.508265156028171 | 6 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | hip fracture | targetBased | 394050 | 3624 | 0.560682335264164 | 17 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | hip fracture | targetBased | 335239 | 991 | 0.595650699705387 | 9 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | hip fracture | targetBased | 335239 | 695 | 0.595650699705387 | 9 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | postmenopausal osteoporosis | targetBased | 86095 | 1442 | 0.643509974124573 | 30 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | postmenopausal osteoporosis | targetBased | 86095 | 1151 | 0.643509974124573 | 30 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | postmenopausal osteoporosis | targetBased | 394050 | 3624 | 0.640838273592233 | 36 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | postmenopausal osteoporosis | targetBased | 86095 | 1114 | 0.651434014354781 | 44 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | postmenopausal osteoporosis | targetBased | 405685 | 308 | 0.650220587016059 | 56 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | bone density | targetBased | 86095 | 1442 | 0.544372294829174 | 60 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | bone density | targetBased | 86095 | 1151 | 0.544372294829174 | 60 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | ankylosing spondylitis | targetBased | 217959 | 2390 | 0.673282147689306 | 33 |
| MLPCN ERAP1 Measured in Biochemical System Using Plate Reader - 7016-01_Inhibitor_SinglePoint_HTS_Activity | ERAP1_inhibitors | ankylosing spondylitis | targetBased | 335777 | 499 | 0.531074016498844 | 207 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | bone fracture | targetBased | 394050 | 3624 | 0.565035984166105 | 29 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | bone fracture | targetBased | 335239 | 991 | 0.63093617484398 | 26 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | bone fracture | targetBased | 335239 | 695 | 0.63093617484398 | 26 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | bone fracture | targetBased | 405685 | 308 | 0.626471657003301 | 21 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | bone fracture | targetBased | 316642 | 617 | 0.557765397120809 | 12 |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | bone fracture | targetBased | 339772 | 8480 | 0.591034537873092 | 14 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | humerus fracture | targetBased | 335239 | 991 | 0.577168330395272 | 6 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | humerus fracture | targetBased | 335239 | 695 | 0.577168330395272 | 6 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | humerus fracture | targetBased | 316642 | 617 | 0.577168330395272 | 6 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | bone disease | targetBased | 86095 | 1442 | 0.518297504276273 | 499 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | bone disease | targetBased | 86095 | 1151 | 0.518297504276273 | 499 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | scoliosis | targetBased | 335239 | 991 | 0.581086621208305 | 17 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | scoliosis | targetBased | 335239 | 695 | 0.581086621208305 | 17 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | gout | pathwayBased | 362051 | 17187 | 0.57690435150965 | 63 |
| HCS assay for microtubule stabilizers | TUBB | gout | targetBased | 195821 | 1625 | 0.650695824149268 | 48 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | bone quantitative ultrasound measurement | targetBased | 86095 | 1442 | 0.553003830357957 | 41 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | bone quantitative ultrasound measurement | targetBased | 86095 | 1151 | 0.553003830357957 | 41 |
| HTS of Smad transcription factor inhibitors | SMAD3 | bone quantitative ultrasound measurement | targetBased | 88033 | 251 | 0.501865676168539 | 11 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | osteoarthritis, knee | targetBased | 394050 | 3624 | 0.579400705699943 | 14 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis, knee | targetBased | 335239 | 991 | 0.644836374558565 | 48 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis, knee | targetBased | 335239 | 695 | 0.644836374558565 | 48 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | osteoarthritis, knee | targetBased | 339297 | 1446 | 0.611730200008939 | 12 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | osteoarthritis, knee | targetBased | 316642 | 617 | 0.615751427985334 | 35 |
| Small Molecule Inhibitors of FGF22-Mediated Excitatory Synaptogenesis & Epilepsy Measured in Biochemical System Using RT-PCR - 7012-01_Inhibitor_SinglePoint_HTS_Activity | FGF22_inhibitors | bone development disease | targetBased | 339650 | 5128 | 0.628351401578255 | 15 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | bone development disease | targetBased | 369953 | 3123 | 0.607202180524209 | 10 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | rickets | targetBased | 394050 | 3624 | 0.588911011139894 | 107 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | polycythemia | targetBased | 217959 | 2390 | 0.547877540544829 | 613 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | fibromyalgia | targetBased | 363803 | 2412 | 0.615589784440467 | 32 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | fibromyalgia | targetBased | 335239 | 991 | 0.505730515267723 | 51 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | fibromyalgia | targetBased | 335239 | 695 | 0.505730515267723 | 51 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | fibromyalgia | targetBased | 359518 | 300 | 0.567270683537583 | 15 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | fibromyalgia | targetBased | 335652 | 1779 | 0.567270683537583 | 15 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | fibromyalgia | targetBased | 357537 | 806 | 0.567270683537583 | 15 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | fibromyalgia | targetBased | 339887 | 1178 | 0.567270683537583 | 15 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | fibromyalgia | targetBased | 362274 | 1056 | 0.567270683537583 | 15 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | fibromyalgia | targetBased | 336308 | 6862 | 0.567270683537583 | 15 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | arthritis | targetBased | 316642 | 617 | 0.651046057163841 | 78 |
| Primary screen for KDM5B Histone Demethylases FDH-coupled qHTS | KDM5B | grip strength measurement | targetBased | 217819 | 5697 | 0.522906509080935 | 35 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | sinusitis | targetBased | 339297 | 1446 | 0.637275760427135 | 26 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | Fanconi anemia complementation group A | pathwayBased | 376029 | 3978 | 0.620838562759111 | 6 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | heel bone mineral density | targetBased | 86095 | 1442 | 0.559275327168255 | 59 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | heel bone mineral density | targetBased | 86095 | 1151 | 0.559275327168255 | 59 |
| HTS of Smad transcription factor inhibitors | SMAD3 | heel bone mineral density | targetBased | 88033 | 251 | 0.547319979971764 | 24 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | rib fracture | targetBased | 335239 | 991 | 0.601285568397495 | 9 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | rib fracture | targetBased | 335239 | 695 | 0.601285568397495 | 9 |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | rib fracture | targetBased | 339772 | 8480 | 0.569561257660925 | 6 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | musculoskeletal system disease | targetBased | 339297 | 1446 | 0.514238155627858 | 99 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | toothache | targetBased | 335239 | 991 | 0.532764875117537 | 11 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | toothache | targetBased | 335239 | 695 | 0.532764875117537 | 11 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | toothache | targetBased | 316642 | 617 | 0.644988505060209 | 38 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | muscle cramp | targetBased | 316642 | 617 | 0.619285523949174 | 12 |
| uHTS identification of TNAP inhibitors in the absence of phosphate acceptor performed in luminescent assay | ALPL_inhibitors | adult hypophosphatasia | targetBased | 195560 | 517 | 0.939215261483421 | 551 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | rotator cuff tear | targetBased | 290355 | 265 | 0.527975236729244 | 11 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | rotator cuff tear | targetBased | 335239 | 991 | 0.567119417340278 | 15 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | rotator cuff tear | targetBased | 335239 | 695 | 0.567119417340278 | 15 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis, hip | targetBased | 335239 | 991 | 0.61595213184444 | 18 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis, hip | targetBased | 335239 | 695 | 0.61595213184444 | 18 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | pulpitis | targetBased | 335239 | 991 | 0.524471038386675 | 9 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | pulpitis | targetBased | 335239 | 695 | 0.524471038386675 | 9 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | pulpitis | targetBased | 339297 | 1446 | 0.586098907288342 | 12 |
| HTS of Smad transcription factor inhibitors | SMAD3 | connective tissue disease | targetBased | 88033 | 251 | 0.63854754175007 | 547 |
| Luminescence-based cell-based primary high throughput screening assay to identify biased ligands of the melanocortin 4 receptor (MC4R): agonists of MC4R | MC4R | Abnormality of the skeletal system | targetBased | 356160 | 3470 | 0.654264744820366 | 73 |
| TRFRET-based cell-based primary high throughput screening assay to identify biased ligands of the melanocortin 4 receptor (MC4R): antagonists of MC4R | MC4R | Abnormality of the skeletal system | targetBased | 356160 | 1703 | 0.654264744820366 | 73 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | Osteopenia | targetBased | 394050 | 3624 | 0.57648010585294 | 47 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | Osteopenia | targetBased | 86095 | 1114 | 0.556646138618613 | 8 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | Abnormal skeletal morphology | targetBased | 359207 | 1432 | 0.550489496986252 | 13 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Sciatica | targetBased | 335239 | 991 | 0.52560928186248 | 6 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Sciatica | targetBased | 335239 | 695 | 0.52560928186248 | 6 |
| uHTS identification of TNAP inhibitors in the absence of phosphate acceptor performed in luminescent assay | ALPL_inhibitors | Skeletal dysplasia | targetBased | 195560 | 517 | 0.624363486174286 | 11 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | Muscle spasm | targetBased | 363803 | 2412 | 0.60466648961276 | 9 |
| High-throughput screen for inhibitors of the GIV GBA-motif interaction with Galpha-i G protein subunits | GNAI3 | auriculocondylar syndrome | targetBased | 204125 | 876 | 0.791157912368328 | 41 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | bone marrow failure syndrome | pathwayBased | 321427 | 201 | 0.737460111393103 | 73 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bone marrow failure syndrome | targetBased | 54509 | 528 | 0.737460111393103 | 73 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bone marrow failure syndrome | targetBased | 54513 | 338 | 0.737460111393103 | 73 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bone marrow failure syndrome | targetBased | 125394 | 1890 | 0.737460111393103 | 73 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bone marrow failure syndrome | targetBased | 124022 | 1156 | 0.737460111393103 | 73 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | osteoarthritis | targetBased | 290355 | 265 | 0.629392602569647 | 41 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis | targetBased | 335239 | 991 | 0.657280791256667 | 97 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | osteoarthritis | targetBased | 335239 | 695 | 0.657280791256667 | 97 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | osteoarthritis | targetBased | 339297 | 1446 | 0.581476497553818 | 17 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | osteoarthritis | targetBased | 316642 | 617 | 0.645932652943565 | 116 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | cardiomyopathy, familial restrictive, 1 | targetBased | 335239 | 801 | 0.839628176662135 | 108 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | cardiomyopathy, familial restrictive, 1 | targetBased | 335238 | 390 | 0.839628176662135 | 108 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | isolated congenital digital clubbing | targetBased | 148480 | 6428 | 0.710106043082438 | 116 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | familial cold autoinflammatory syndrome 1 | targetBased | 330392 | 1295 | 0.873832417903922 | 297 |
| qHTS for Inhibitors of TGF-b | TGFB1 | Camurati-Engelmann disease | pathwayBased | 403345 | 4970 | 0.882045406274388 | 110 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | Acrodysostosis 1 with or without hormone resistance | targetBased | 343468 | 285 | 0.829085639795519 | 192 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudohypoparathyroidism type 1A | targetBased | 334825 | 423 | 0.931191434735326 | 489 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudohypoparathyroidism type 1A | targetBased | 337446 | 1356 | 0.931191434735326 | 489 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | systemic lupus erythematosus | targetBased | 343468 | 734 | 0.606714042774157 | 24 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | Blau syndrome | targetBased | 292323 | 1836 | 0.939286992074845 | 1956 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | metaphyseal chondrodysplasia, Jansen type | targetBased | 405685 | 308 | 0.870699161633779 | 171 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | Muckle-Wells syndrome | pathwayBased | 362051 | 17187 | 0.543319270372889 | 54 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | Muckle-Wells syndrome | targetBased | 330392 | 1295 | 0.894883848273049 | 515 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | amelogenesis imperfecta type 1G | targetBased | 343468 | 285 | 0.598819185739921 | 44 |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit/block inward-rectifying potassium ion channel Kir2.1 | KCNJ2 | Andersen-Tawil syndrome | targetBased | 305610 | 2592 | 0.928674186301643 | 1100 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | polydactyly of a biphalangeal thumb | targetBased | 338328 | 2501 | 0.706647047561123 | 14 |
| Glucocerebrosidase | GBA1 | Gaucher disease type III | targetBased | 48118 | 549 | 0.843925331047856 | 164 |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | glycogen storage disease II | targetBased | 199169 | 715 | 0.960121004133098 | 4311 |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | glycogen storage disease II | targetBased | 302297 | 1165 | 0.960121004133098 | 4311 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | Proteus syndrome | targetBased | 356517 | 1139 | 0.869235310377157 | 179 |
| Primary cell-based high-throughput screening assay to measure PERK inhibition | PERK_inhibitors | Wolcott-Rallison syndrome | targetBased | 217959 | 370 | 0.873386417124152 | 435 |
| Total Fluorescence Counterscreen for Inhibitors of the Interaction of Thyroid Hormone Receptor and Steroid Receptor Coregulator 2 | THRB | glycogen storage disease due to glycogen branching enzyme deficiency | targetBased | 276265 | 806 | 0.758107361832069 | 338 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | chondrodysplasia Blomstrand type | targetBased | 405685 | 308 | 0.824076159020484 | 170 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | Costello syndrome | targetBased | 194628 | 267 | 0.971682105449753 | 1184 |
| Primary Cell-based High Throughput Screening assay for activators of the nuclear receptor Steroidogenic Factor 1 (SF-1) | NR5A1 | 46,XY complete gonadal dysgenesis | targetBased | 64908 | 1225 | 0.579738241896345 | 77 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the nuclear receptor Steroidogenic Factor 1 (SF-1) | NR5A1 | 46,XY complete gonadal dysgenesis | targetBased | 64908 | 359 | 0.579738241896345 | 77 |
| Luminescence-based cell-based primary high throughput screening assay for inhibitors of the orphan nuclear receptor subfamily 0, group B, member 1 (DAX1; NR0B1): repression of SF-1 (NR5A1) activated StAR promoter by full-length DAX-1 | NR0B1 | 46,XY complete gonadal dysgenesis | targetBased | 368927 | 4094 | 0.714510258851383 | 372 |
| Luminescence-based primary cell-based high throughput screening assay to identify inhibitors of the orphan nuclear receptor subfamily 0, group B, member 1 (DAX1; NR0B1) | NR0B1 | 46,XY complete gonadal dysgenesis | targetBased | 343468 | 3417 | 0.714510258851383 | 372 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | Eiken syndrome | targetBased | 405685 | 308 | 0.765164840016023 | 86 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | Weaver syndrome | targetBased | 57013 | 201 | 0.907269836964628 | 782 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Duchenne muscular dystrophy | targetBased | 339297 | 1446 | 0.514857952397938 | 8 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | sickle cell anemia | targetBased | 335239 | 991 | 0.631524990132642 | 24 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | sickle cell anemia | targetBased | 335239 | 695 | 0.631524990132642 | 24 |
| qHTS Assay for Inhibitors of the CtBP/E1A Interaction | RBBP8 | Seckel syndrome 2 | targetBased | 335214 | 1652 | 0.795909043926353 | 35 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | erythrocytosis, familial, 4 | targetBased | 363840 | 2624 | 0.779929786051706 | 233 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | CINCA syndrome | targetBased | 330392 | 1295 | 0.925563914784182 | 621 |
| HTS-Luminescent assay for inhibitors of ALR by detection of hydrogen peroxide production Measured in Biochemical System Using Plate Reader - 2036-02_Inhibitor_SinglePoint_HTS | ALR inhibitors | congenital cataract-progressive muscular hypotonia-hearing loss-developmental delay syndrome | targetBased | 288728 | 10857 | 0.770329660937942 | 30 |
| HTS-Luminescent assay for inhibitors of ALR by detection of hydrogen peroxide production Measured in Biochemical System Using Plate Reader - 2036-02_Inhibitor_SinglePoint_HTS | ALR activators | congenital cataract-progressive muscular hypotonia-hearing loss-developmental delay syndrome | targetBased | 288728 | 10857 | 0.770329660937942 | 30 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy 2A | targetBased | 335239 | 801 | 0.744453709686017 | 86 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy 2A | targetBased | 335238 | 390 | 0.744453709686017 | 86 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy 1FF | targetBased | 335239 | 801 | 0.830166550514321 | 63 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | dilated cardiomyopathy 1FF | targetBased | 335238 | 390 | 0.830166550514321 | 63 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudohypoparathyroidism type 1C | targetBased | 334825 | 423 | 0.900125093342387 | 147 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudohypoparathyroidism type 1C | targetBased | 337446 | 1356 | 0.900125093342387 | 147 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudopseudohypoparathyroidism | targetBased | 334825 | 423 | 0.857715919055999 | 266 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | pseudopseudohypoparathyroidism | targetBased | 337446 | 1356 | 0.857715919055999 | 266 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | hypertrophic cardiomyopathy 7 | targetBased | 335239 | 801 | 0.885049175732636 | 144 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | hypertrophic cardiomyopathy 7 | targetBased | 335238 | 390 | 0.885049175732636 | 144 |
| HTS of Smad transcription factor inhibitors | SMAD3 | aneurysm-osteoarthritis syndrome | targetBased | 88033 | 251 | 0.88651169542596 | 492 |
| qHTS assay for inhibitors of human lactate dehydrogenase | lactateDehydrogenaseInhibitors | glycogen storage disease due to lactate dehydrogenase M-subunit deficiency | targetBased | 476056 | 732 | 0.825949553451582 | 236 |
| Primary cell-based screen for identification of compounds that inhibit the Choline Transporter (CHT) | SLC5A7 | distal hereditary motor neuropathy type 7 | targetBased | 306502 | 2634 | 0.632249836516357 | 623 |
| Primary cell-based screen for identification of compounds that allosterically activate the Choline Transporter (CHT) | SLC5A7 | distal hereditary motor neuropathy type 7 | targetBased | 306502 | 1509 | 0.632249836516357 | 623 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | familial restrictive cardiomyopathy | targetBased | 335239 | 801 | 0.526611777023384 | 59 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | familial restrictive cardiomyopathy | targetBased | 335238 | 390 | 0.526611777023384 | 59 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | Coffin-Siris syndrome | targetBased | 344733 | 7043 | 0.76887560431398 | 1260 |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit/block inward-rectifying potassium ion channel Kir2.1 | KCNJ2 | periodic paralysis | targetBased | 305610 | 2592 | 0.68356093063422 | 449 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | cryopyrin-associated periodic syndrome | pathwayBased | 362051 | 17187 | 0.605771408191478 | 546 |
| QFRET-based biochemical primary high throughput screening assay to identify exosite inhibitors of ADAM17. | ADAM17_inhibitors | neonatal inflammatory skin and bowel disease | targetBased | 369953 | 3080 | 0.78132175077845 | 681 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | preaxial polydactyly of fingers | targetBased | 338328 | 2501 | 0.514204542415849 | 6 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | cryopyrin-associated periodic syndrome | targetBased | 330392 | 1295 | 0.74279650622679 | 2442 |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | glycogen storage disease due to acid maltase deficiency, infantile onset | targetBased | 199169 | 715 | 0.642982582442733 | 17 |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | glycogen storage disease due to acid maltase deficiency, infantile onset | targetBased | 302297 | 1165 | 0.642982582442733 | 17 |
| HCS assay for microtubule stabilizers | TUBB | familial Mediterranean fever | targetBased | 195821 | 1625 | 0.637516775910312 | 23 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | familial cold autoinflammatory syndrome | targetBased | 330392 | 1295 | 0.595263504725513 | 254 |
| RNA aptamer-based HTS for inhibitors of GRK2 | GRK2 | Jeune syndrome | targetBased | 327939 | 1924 | 0.522237752095036 | 12 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | osteogenesis imperfecta | targetBased | 394050 | 3624 | 0.527327245164823 | 65 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | osteogenesis imperfecta | targetBased | 405685 | 308 | 0.504678620737738 | 61 |
| uHTS identification of TNAP inhibitors in the absence of phosphate acceptor performed in luminescent assay | ALPL_inhibitors | osteogenesis imperfecta | targetBased | 195560 | 517 | 0.590018585582101 | 59 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | McCune-Albright syndrome | targetBased | 334825 | 423 | 0.895970037054108 | 329 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | McCune-Albright syndrome | targetBased | 337446 | 1356 | 0.895970037054108 | 329 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | Fanconi anemia | pathwayBased | 376029 | 3978 | 0.57760274438034 | 421 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | AL amyloidosis | targetBased | 143816 | 859 | 0.524188480440994 | 22 |
| HTS of Smad transcription factor inhibitors | SMAD3 | Loeys-Dietz syndrome | targetBased | 88033 | 251 | 0.782631949327588 | 824 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | Noonan syndrome | pathwayBased | 70898 | 707 | 0.735833813629442 | 26 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | Noonan syndrome | pathwayBased | 131324 | 544 | 0.735833813629442 | 26 |
| Assay for Inhibitors of the beta-Arrestin-Adaptor Protein 2 Interaction That Mediate GPCR Degradation and Recycling | ARRB1_inhibitors | Noonan syndrome | targetBased | 338373 | 1061 | 0.577168330395272 | 6 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn6) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | Noonan syndrome | targetBased | 369953 | 760 | 0.577168330395272 | 6 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn7) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | Noonan syndrome | targetBased | 369953 | 498 | 0.577168330395272 | 6 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | Noonan syndrome | targetBased | 194628 | 267 | 0.750196204718814 | 41 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | Noonan syndrome and Noonan-related syndrome | targetBased | 194628 | 267 | 0.704710908817553 | 459 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | autoinflammatory syndrome | targetBased | 330392 | 1295 | 0.702039458685415 | 1491 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | autoinflammatory syndrome | targetBased | 292323 | 1836 | 0.564415235811097 | 914 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | acrodysostosis | targetBased | 343468 | 285 | 0.781959384313481 | 152 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | hypertrophic osteoarthropathy, primary, autosomal recessive, 1 | targetBased | 148480 | 6428 | 0.798213557362017 | 135 |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | familial hypertrophic cardiomyopathy | targetBased | 335239 | 801 | 0.677983200454697 | 73 |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | familial hypertrophic cardiomyopathy | targetBased | 335238 | 390 | 0.677983200454697 | 73 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | postaxial polydactyly type A | targetBased | 338328 | 2501 | 0.523502780989542 | 13 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | polydactyly, postaxial, type A8 | targetBased | 338328 | 2501 | 0.732801265040584 | 21 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | bone marrow failure syndrome 6 | targetBased | 331671 | 10022 | 0.513043524679079 | 6 |
| Fluorescence-based primary biochemical high throughput screening assay to identify inhibitors of human diacylglycerol lipase, beta (DAGLB) | DAGLB_inhibitors | osteogenesis imperfecta, type 21 | targetBased | 343468 | 202 | 0.546808807855395 | 11 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | Fanconi anemia, complementation group S | pathwayBased | 376029 | 3978 | 0.882895059808289 | 251 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | myelofibrosis | targetBased | 217959 | 2390 | 0.707709957175985 | 1089 |
| Luminescence-based cell-based primary high throughput screening assay to identify activators of the GAA850 frataxin (FXN) promoter | FXN | Friedreich ataxia | targetBased | 356160 | 1985 | 0.890999212816761 | 1874 |
| A quantitative high throughput screen for small molecules that induce DNA re-replication in MCF 10a normal breast cells. | GMNN | Ear-patella-short stature syndrome | targetBased | 343234 | 9888 | 0.76120314187953 | 15 |
| A quantitative high throughput screen for small molecules that induce DNA re-replication in SW480 colon adenocarcinoma cells. | GMNN | Ear-patella-short stature syndrome | targetBased | 342279 | 6233 | 0.76120314187953 | 15 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | Pachydermoperiostosis | targetBased | 148480 | 6428 | 0.552231998408656 | 18 |
| Primary cell-based screen for identification of compounds that inhibit the Choline Transporter (CHT) | SLC5A7 | Congenital myasthenic syndromes | targetBased | 306502 | 2634 | 0.774715316348506 | 860 |
| Primary cell-based screen for identification of compounds that allosterically activate the Choline Transporter (CHT) | SLC5A7 | Congenital myasthenic syndromes | targetBased | 306502 | 1509 | 0.774715316348506 | 860 |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of the prolyl oligopeptidase-like enzyme (PREPL) | PREPL | Congenital myasthenic syndromes | targetBased | 324747 | 2221 | 0.727310482862168 | 891 |
| HTS of Smad transcription factor inhibitors | SMAD3 | Aneurysm - osteoarthritis syndrome | targetBased | 88033 | 251 | 0.818691129384453 | 60 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | Albright hereditary osteodystrophy | targetBased | 334825 | 423 | 0.866673808957289 | 189 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | Albright hereditary osteodystrophy | targetBased | 337446 | 1356 | 0.866673808957289 | 189 |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | Glycogen storage disease due to acid maltase deficiency, adult onset | targetBased | 199169 | 715 | 0.551611095948914 | 11 |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | Glycogen storage disease due to acid maltase deficiency, adult onset | targetBased | 302297 | 1165 | 0.551611095948914 | 11 |
| Glucocerebrosidase | GBA1 | Gaucher disease type 1 | targetBased | 48118 | 549 | 0.904732450777115 | 509 |
| Glucocerebrosidase | GBA1 | Gaucher disease type 3 | targetBased | 48118 | 549 | 0.869364153583014 | 180 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | Hypocalcemic vitamin D-resistant rickets | targetBased | 394050 | 3624 | 0.924912744974669 | 399 |
| HTS-Luminescent assay for inhibitors of ALR by detection of hydrogen peroxide production Measured in Biochemical System Using Plate Reader - 2036-02_Inhibitor_SinglePoint_HTS | ALR inhibitors | Congenital cataract - progressive muscular hypotonia - hearing loss - developmental delay | targetBased | 288728 | 10857 | 0.82172283003768 | 36 |
| HTS-Luminescent assay for inhibitors of ALR by detection of hydrogen peroxide production Measured in Biochemical System Using Plate Reader - 2036-02_Inhibitor_SinglePoint_HTS | ALR activators | Congenital cataract - progressive muscular hypotonia - hearing loss - developmental delay | targetBased | 288728 | 10857 | 0.82172283003768 | 36 |
| Luminescent HTS for small molecule inhibitors of MT1-MMP transcription | MMP14 | Torg-Winchester syndrome | targetBased | 86733 | 537 | 0.633573506874993 | 23 |
| uHTS identification of inhibitors of MT1-MMP activation in a fluoresence assay | MMP14 | Torg-Winchester syndrome | targetBased | 361500 | 222 | 0.633573506874993 | 23 |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | Glycogen storage disease due to acid maltase deficiency | targetBased | 199169 | 715 | 0.916238536169051 | 4712 |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | Glycogen storage disease due to acid maltase deficiency | targetBased | 302297 | 1165 | 0.916238536169051 | 4712 |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit/block inward-rectifying potassium ion channel Kir2.1 | KCNJ2 | Cardiodysrhythmic potassium-sensitive periodic paralysis | targetBased | 305610 | 2592 | 0.896801261242644 | 914 |
| Primary cell-based screen for identification of compounds that inhibit the Choline Transporter (CHT) | SLC5A7 | Presynaptic congenital myasthenic syndromes | targetBased | 306502 | 2634 | 0.822381801512511 | 639 |
| Primary cell-based screen for identification of compounds that allosterically activate the Choline Transporter (CHT) | SLC5A7 | Presynaptic congenital myasthenic syndromes | targetBased | 306502 | 1509 | 0.822381801512511 | 639 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | Blomstrand lethal chondrodysplasia | targetBased | 405685 | 308 | 0.71396083712997 | 35 |
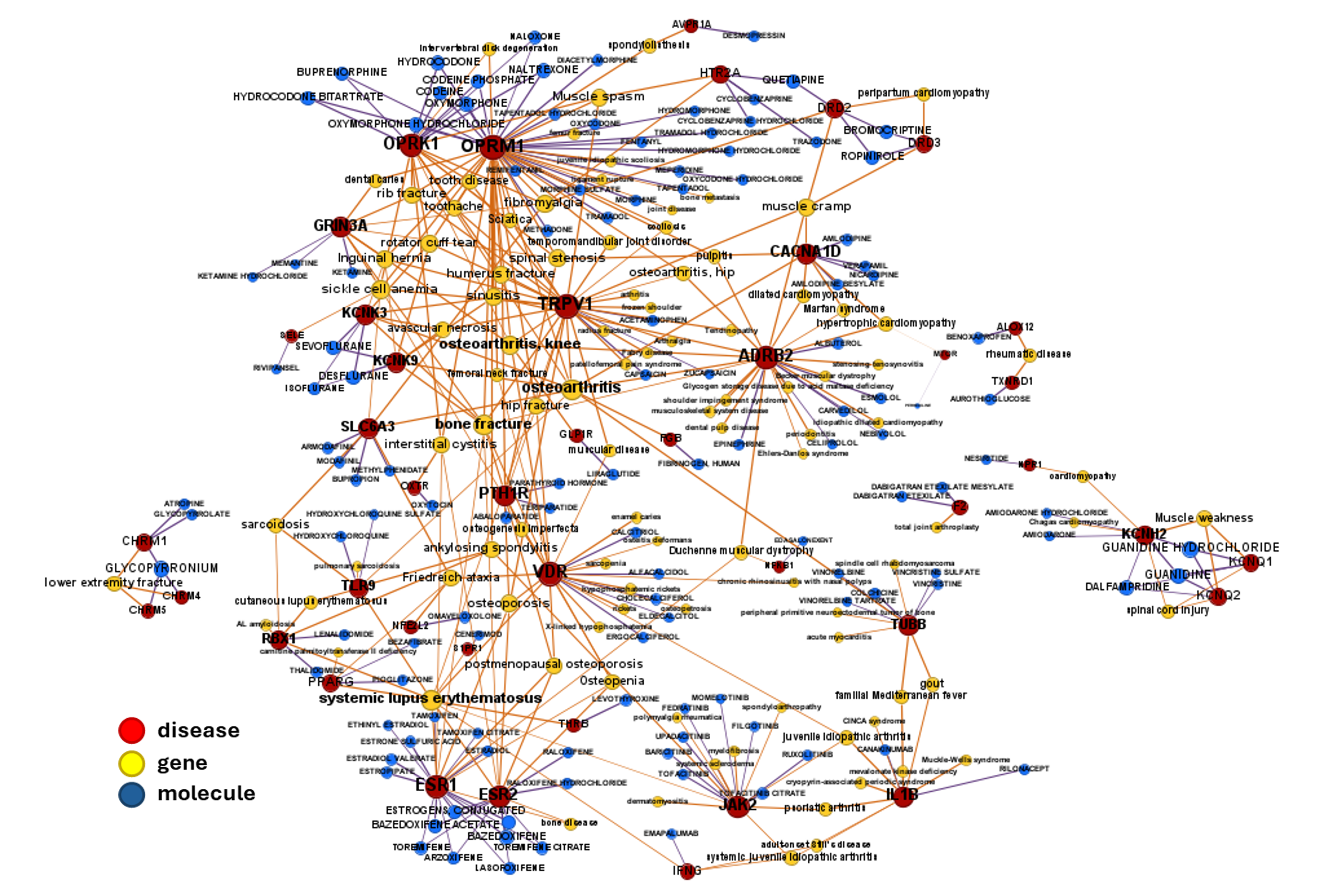
Some of these associations have also gone through clinical trials, as those in the graph below.
Find clinical trials details in table. Use scroll bar or search funtion to select specific drugs, molecular targets or diseases.
| BioAssay Name | program | gene | protName | diseaseName | molname | assayMode | clinicalPhase | clinicalStatus | studyStartDate | url | score | variantEffect | directionOnTrait | studyStopReason |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | hypertrophic cardiomyopathy | VERAPAMIL | targetBased | 4 | Recruiting | 10/08/2022 | https://clinicaltrials.gov/study/NCT05569382 | 1 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | muscle cramp | VERAPAMIL | targetBased | 4 | Recruiting | 05/06/2024 | https://clinicaltrials.gov/study/NCT06447688 | 1 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | Marfan syndrome | VERAPAMIL | targetBased | 4 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT01295047 | 1 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | dilated cardiomyopathy | VERAPAMIL | targetBased | 4 | Unknown status | 01/01/2006 | https://clinicaltrials.gov/study/NCT00374465 | 1 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | hypertrophic cardiomyopathy | DILTIAZEM | targetBased | 2 | Completed | 01/01/2006 | https://clinicaltrials.gov/study/NCT00319982 | 0.2 | LoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | ankylosing spondylitis | OLSALAZINE | targetBased | 2 | Completed | 01/05/1996 | https://clinicaltrials.gov/study/NCT00004288 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sickle cell anemia | PROPRANOLOL | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01077921 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Marfan syndrome | PROPRANOLOL | targetBased | 2 | Unknown status | 01/02/2007 | https://clinicaltrials.gov/study/NCT00651235 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | LABETALOL | targetBased | 2 | Completed | 01/03/2017 | https://clinicaltrials.gov/study/NCT03661346 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | myelofibrosis | THALIDOMIDE | targetBased | 2 | Active, not recruiting | 27/02/2017 | https://clinicaltrials.gov/study/NCT03069326 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | myelofibrosis | THALIDOMIDE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00445900 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | systemic lupus erythematosus | THALIDOMIDE | targetBased | 4 | Completed | 05/06/2017 | https://clinicaltrials.gov/study/NCT03122431 | 1 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | primary systemic amyloidosis | THALIDOMIDE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT01527032 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | sarcoidosis | THALIDOMIDE | targetBased | 3 | Completed | 01/02/2005 | https://clinicaltrials.gov/study/NCT00305552 | 0.7 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | cutaneous lupus erythematosus | THALIDOMIDE | targetBased | 4 | Completed | 05/06/2017 | https://clinicaltrials.gov/study/NCT03122431 | 1 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | ankylosing spondylitis | THALIDOMIDE | targetBased | 4 | Active, not recruiting | 15/03/2022 | https://clinicaltrials.gov/study/NCT05527444 | 1 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | ankylosing spondylitis | THALIDOMIDE | targetBased | 2 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT02201043 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | discoid lupus erythematosus | THALIDOMIDE | targetBased | 2 | Completed | 01/10/1997 | https://clinicaltrials.gov/study/NCT00001680 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | THALIDOMIDE | targetBased | 3 | Terminated | 26/12/2012 | https://clinicaltrials.gov/study/NCT01659658 | 0.7 | LoF | protect | Sponsor's decision |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | THALIDOMIDE | targetBased | 4 | Recruiting | 01/01/2020 | https://clinicaltrials.gov/study/NCT04612582 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Intervertebral disk degeneration | MORPHINE | targetBased | 4 | Completed | 01/11/2005 | https://clinicaltrials.gov/study/NCT00353704 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Intervertebral disk degeneration | MORPHINE | targetBased | 4 | Completed | 01/11/2005 | https://clinicaltrials.gov/study/NCT00353704 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | MORPHINE | targetBased | 4 | Unknown status | 01/01/2015 | https://clinicaltrials.gov/study/NCT02504996 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | MORPHINE | targetBased | 4 | Unknown status | 01/01/2015 | https://clinicaltrials.gov/study/NCT02504996 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | MORPHINE | targetBased | 4 | Unknown status | 01/09/2009 | https://clinicaltrials.gov/study/NCT01281891 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | MORPHINE | targetBased | 4 | Unknown status | 01/09/2009 | https://clinicaltrials.gov/study/NCT01281891 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femur fracture | MORPHINE | targetBased | 4 | Recruiting | 04/09/2023 | https://clinicaltrials.gov/study/NCT05920642 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femur fracture | MORPHINE | targetBased | 4 | Recruiting | 04/09/2023 | https://clinicaltrials.gov/study/NCT05920642 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 3 | Terminated | 22/06/2018 | https://clinicaltrials.gov/study/NCT03537612 | 0.7 | GoF | protect | Do to our inability to meet recruitment targets - Sponsor stopped funding. Looking for new sponsor and protocol modifications |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 3 | Terminated | 22/06/2018 | https://clinicaltrials.gov/study/NCT03537612 | 0.7 | GoF | protect | Do to our inability to meet recruitment targets - Sponsor stopped funding. Looking for new sponsor and protocol modifications |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00737997 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00737997 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MORPHINE | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 4 | Terminated | 01/02/2009 | https://clinicaltrials.gov/study/NCT00880529 | 1 | GoF | protect | low enrollment, 2 pts enrolled no data every analyzed investigator left the institution |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 4 | Terminated | 01/02/2009 | https://clinicaltrials.gov/study/NCT00880529 | 1 | GoF | protect | low enrollment, 2 pts enrolled no data every analyzed investigator left the institution |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 3 | Terminated | 08/08/2016 | https://clinicaltrials.gov/study/NCT02749409 | 0.7 | GoF | protect | Case numbers not enough, however, the funding is over |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | MORPHINE | targetBased | 3 | Terminated | 08/08/2016 | https://clinicaltrials.gov/study/NCT02749409 | 0.7 | GoF | protect | Case numbers not enough, however, the funding is over |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | MORPHINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | MORPHINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | multiple bone fractures | MORPHINE | targetBased | 2 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01807429 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | multiple bone fractures | MORPHINE | targetBased | 2 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01807429 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE | targetBased | 4 | Withdrawn | 01/03/2016 | https://clinicaltrials.gov/study/NCT02698228 | 1 | GoF | protect | Study did not occur |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE | targetBased | 4 | Withdrawn | 01/03/2016 | https://clinicaltrials.gov/study/NCT02698228 | 1 | GoF | protect | Study did not occur |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT03693404 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT03693404 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femoral neck fracture | MORPHINE | targetBased | 4 | Unknown status | 01/09/2010 | https://clinicaltrials.gov/study/NCT01219088 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femoral neck fracture | MORPHINE | targetBased | 4 | Unknown status | 01/09/2010 | https://clinicaltrials.gov/study/NCT01219088 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femoral neck fracture | MORPHINE | targetBased | 4 | Terminated | 01/09/2010 | https://clinicaltrials.gov/study/NCT01219062 | 1 | GoF | protect | Surgeon team were not happy with the study protocol, the periarticular injection of local anesthetics |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | femoral neck fracture | MORPHINE | targetBased | 4 | Terminated | 01/09/2010 | https://clinicaltrials.gov/study/NCT01219062 | 1 | GoF | protect | Surgeon team were not happy with the study protocol, the periarticular injection of local anesthetics |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE | targetBased | 4 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02689024 | 1 | GoF | protect | recruitment too slow; intervention was standard care in patients who were not included; acute care pathways changed due to policy regarding hip fracture patients |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE | targetBased | 4 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02689024 | 1 | GoF | protect | recruitment too slow; intervention was standard care in patients who were not included; acute care pathways changed due to policy regarding hip fracture patients |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01904071 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01904071 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Not yet recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06042426 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Not yet recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06042426 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00901628 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00901628 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00562627 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00562627 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 2 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02860949 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 2 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02860949 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 2 | Unknown status | 01/08/2015 | https://clinicaltrials.gov/study/NCT02967302 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 2 | Unknown status | 01/08/2015 | https://clinicaltrials.gov/study/NCT02967302 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Withdrawn | 01/02/2016 | https://clinicaltrials.gov/study/NCT02765815 | 1 | GoF | protect | competing protocol |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE | targetBased | 4 | Withdrawn | 01/02/2016 | https://clinicaltrials.gov/study/NCT02765815 | 1 | GoF | protect | competing protocol |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | temporomandibular joint disorder | MORPHINE | targetBased | 4 | Completed | 10/12/2013 | https://clinicaltrials.gov/study/NCT04110587 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | temporomandibular joint disorder | MORPHINE | targetBased | 4 | Completed | 10/12/2013 | https://clinicaltrials.gov/study/NCT04110587 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | MORPHINE | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | MORPHINE | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | MORPHINE | targetBased | 4 | Completed | 01/05/2016 | https://clinicaltrials.gov/study/NCT02658149 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | MORPHINE | targetBased | 4 | Completed | 01/05/2016 | https://clinicaltrials.gov/study/NCT02658149 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | MORPHINE | targetBased | 4 | Completed | 01/11/2014 | https://clinicaltrials.gov/study/NCT02292082 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | MORPHINE | targetBased | 4 | Completed | 01/11/2014 | https://clinicaltrials.gov/study/NCT02292082 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 2 | Completed | 01/11/2019 | https://clinicaltrials.gov/study/NCT04301336 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 2 | Completed | 01/11/2019 | https://clinicaltrials.gov/study/NCT04301336 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00513864 | 1 | GoF | protect | lack of funding |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 4 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00513864 | 1 | GoF | protect | lack of funding |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 3 | Terminated | 13/08/2019 | https://clinicaltrials.gov/study/NCT03933397 | 0.7 | GoF | protect | Due to COVID enrollment numbers needed to meet the primary endpoint will not be met. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE | targetBased | 3 | Terminated | 13/08/2019 | https://clinicaltrials.gov/study/NCT03933397 | 0.7 | GoF | protect | Due to COVID enrollment numbers needed to meet the primary endpoint will not be met. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Sciatica | CODEINE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | tooth disease | CODEINE | targetBased | 4 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02547896 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | tooth disease | CODEINE | targetBased | 4 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02547896 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | CODEINE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | CODEINE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02501564 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | CODEINE | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Muscle spasm | CODEINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | toothache | CODEINE | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00694369 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Muscle spasm | CODEINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Muscle spasm | CODEINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CODEINE | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00264225 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CODEINE | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00264225 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | sickle cell anemia | CODEINE | targetBased | 4 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00513864 | 1 | GoF | protect | lack of funding |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis, knee | CODEINE | targetBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00264225 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | tooth disease | CODEINE | targetBased | 4 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02547896 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02501564 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | CODEINE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02501564 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | CODEINE | targetBased | 4 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00513864 | 1 | GoF | protect | lack of funding |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | CODEINE | targetBased | 4 | Withdrawn | 01/11/2006 | https://clinicaltrials.gov/study/NCT00513864 | 1 | GoF | protect | lack of funding |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | CODEINE | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00694369 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | CODEINE | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00694369 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | NALOXONE | targetBased | 3 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00902837 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | NALOXONE | targetBased | 3 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00902837 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | NALOXONE | targetBased | 3 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00902837 | 0.7 | LoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | Osteopenia | RALOXIFENE | targetBased | 4 | Completed | 31/07/2000 | https://clinicaltrials.gov/study/NCT00431431 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | Osteopenia | RALOXIFENE | targetBased | 3 | Completed | 01/02/2004 | https://clinicaltrials.gov/study/NCT00310531 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | Osteopenia | RALOXIFENE | targetBased | 4 | Completed | 16/12/2020 | https://clinicaltrials.gov/study/NCT05386784 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00046137 | 0.7 | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Active, not recruiting | 30/11/2018 | https://clinicaltrials.gov/study/NCT03623633 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00079924 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 3 | Completed | 02/04/2001 | https://clinicaltrials.gov/study/NCT00389740 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 3 | Completed | 01/11/1994 | https://clinicaltrials.gov/study/NCT00670319 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/09/2009 | https://clinicaltrials.gov/study/NCT01544894 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00532246 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00383422 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/08/2002 | https://clinicaltrials.gov/study/NCT00191425 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/10/2001 | https://clinicaltrials.gov/study/NCT00035256 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/evista | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Terminated | 01/06/2004 | https://clinicaltrials.gov/study/NCT00790101 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00532428 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT00371956 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Active, not recruiting | 30/11/2018 | https://clinicaltrials.gov/study/NCT03623633 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Completed | https://clinicaltrials.gov/study/NCT00035971 | 1 | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fcaaa6dc-74e8-4fb8-800c-5574bf0f8de9 | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT01535027 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01166958 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 4 | Completed | 01/01/2007 | https://clinicaltrials.gov/study/NCT00431444 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE | targetBased | 3 | Completed | 01/05/2003 | https://clinicaltrials.gov/study/NCT00163137 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | Duchenne muscular dystrophy | TAMOXIFEN | targetBased | 3 | Completed | 12/06/2018 | https://clinicaltrials.gov/study/NCT03354039 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | Duchenne muscular dystrophy | TAMOXIFEN | targetBased | 3 | Completed | 12/06/2018 | https://clinicaltrials.gov/study/NCT03354039 | 0.7 | protect | ||
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 2 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00998556 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 3 | Withdrawn | 01/01/2017 | https://clinicaltrials.gov/study/NCT02590601 | 0.7 | GoF | protect | Not enough patients |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peripartum cardiomyopathy | BROMOCRIPTINE | targetBased | 4 | Recruiting | 27/07/2022 | https://clinicaltrials.gov/study/NCT05180773 | 1 | GoF | protect | |
| Fluorescence Cell-Free Homogeneous Primary HTS to Identify Inhibitors of Histone Deacetylase 3 | HDAC3 | HDAC3 | Histone deacetylase 3 | sickle cell anemia | VORINOSTAT | targetBased | 2 | Terminated | 01/10/2009 | https://clinicaltrials.gov/study/NCT01000155 | 0.2 | LoF | protect | The study terminated early due to slow accrual. |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | osteoarthritis, knee | COLCHICINE | targetBased | 2 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT02176460 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | acute myocarditis | COLCHICINE | targetBased | 3 | Not yet recruiting | 22/06/2024 | https://clinicaltrials.gov/study/NCT05855746 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 2 | Unknown status | 01/06/2014 | https://clinicaltrials.gov/study/NCT02175589 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d6adc880-5499-4691-93f3-27c87902d5fc | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95a7ff27-b165-436a-b160-7a0f8ec95d7d | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=847af9a9-57e3-4474-99ab-89cd0380f125 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96ca32da-68cc-d757-e053-2995a90a76d8 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT02602028 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4e936802-5b5d-4772-8b1d-4f30867ffa59 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=153f5af8-827e-4a58-a0bd-5aafcb3b5444 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ecaa34e-1bed-49a5-b97c-a8f8c0d885a7 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c7d013b7-0a67-0953-e053-2a95a90ac47c | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=14d4be16-d355-4baf-b4cd-4d97d4b2dca0 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=56c130d1-7581-4152-99a2-0014ee9366c0 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a0688f33-f00f-4a68-abc3-73ec887a471a | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | familial Mediterranean fever | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a80036fe-3016-4541-aa42-6c06cf37ae55 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a80036fe-3016-4541-aa42-6c06cf37ae55 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00819585 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Recruiting | 30/06/2023 | https://clinicaltrials.gov/study/NCT05936268 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Recruiting | 18/01/2023 | https://clinicaltrials.gov/study/NCT05698680 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 3 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00506883 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=847af9a9-57e3-4474-99ab-89cd0380f125 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a0567fe6-132b-463b-bc46-9f5a2a2478fb | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ecaa34e-1bed-49a5-b97c-a8f8c0d885a7 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=153f5af8-827e-4a58-a0bd-5aafcb3b5444 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Completed | 17/08/2015 | https://clinicaltrials.gov/study/NCT02500641 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4e936802-5b5d-4772-8b1d-4f30867ffa59 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01451645 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a0688f33-f00f-4a68-abc3-73ec887a471a | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02139046 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Completed | 12/02/2020 | https://clinicaltrials.gov/study/NCT04130204 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=M04AC01 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT02060552 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=176af20d-d082-47bd-bc56-a21cc1244a33 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Recruiting | 23/01/2024 | https://clinicaltrials.gov/study/NCT04875702 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 2 | Completed | 01/04/2015 | https://clinicaltrials.gov/study/NCT02330796 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d6adc880-5499-4691-93f3-27c87902d5fc | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ebf556e5-7e01-770c-9dc2-4cdfd661c132 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95a7ff27-b165-436a-b160-7a0f8ec95d7d | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cb5f9d85-6b81-49f8-bcd6-17b7bfbc10f2 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Terminated | 10/09/2019 | https://clinicaltrials.gov/study/NCT03933007 | 1 | LoF | protect | faillure of recrutement |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT01994226 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=56c130d1-7581-4152-99a2-0014ee9366c0 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gout | COLCHICINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ee1583ac-c308-4beb-b602-9ecac4977026 | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | osteoarthritis | COLCHICINE | targetBased | 4 | Completed | 15/05/2019 | https://clinicaltrials.gov/study/NCT03913442 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | osteoarthritis | COLCHICINE | targetBased | 4 | Completed | 15/01/2021 | https://clinicaltrials.gov/study/NCT04601883 | 1 | LoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | tooth disease | ACETAMINOPHEN | targetBased | 4 | Completed | 04/03/2017 | https://clinicaltrials.gov/study/NCT04769557 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, hip | ACETAMINOPHEN | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00240773 | 0.7 | protect | |||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | humerus fracture | ACETAMINOPHEN | targetBased | 4 | Terminated | 07/06/2021 | https://clinicaltrials.gov/study/NCT04905563 | 1 | protect | Clinical evidence supporting the hypothesis of this study has been published | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | humerus fracture | ACETAMINOPHEN | targetBased | 4 | Recruiting | 26/02/2019 | https://clinicaltrials.gov/study/NCT03759028 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | humerus fracture | ACETAMINOPHEN | targetBased | 4 | Enrolling by invitation | 15/04/2019 | https://clinicaltrials.gov/study/NCT06187584 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | humerus fracture | ACETAMINOPHEN | targetBased | 4 | Enrolling by invitation | 12/09/2023 | https://clinicaltrials.gov/study/NCT05640674 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6197eff5-0351-42ef-bb3f-cbc26e6d0d14 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=00eb3e4b-1507-4c9a-83a5-ef50cb312753 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07c4cc3d-9a68-42a1-801d-5d4ec107ffad | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5ea858e-76d9-4777-b3dc-5d614f66d485 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d384e88-5c11-4600-ac6c-a38371570138 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=889c5b6a-879e-425c-a25a-435766e9d601 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=55768b45-fd98-4779-85b6-6a50c628677e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscle cramp | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dad63da3-cd1f-42a8-93d5-44b39aae1268 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | radius fracture | ACETAMINOPHEN | targetBased | 4 | Completed | 02/01/2019 | https://clinicaltrials.gov/study/NCT03749616 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 3 | Completed | 01/02/2006 | https://clinicaltrials.gov/study/NCT00298974 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01699815 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Recruiting | 13/09/2022 | https://clinicaltrials.gov/study/NCT05556356 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00240786 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00240799 | 0.7 | protect | |||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 3 | Withdrawn | 01/11/2013 | https://clinicaltrials.gov/study/NCT01420666 | 0.7 | protect | The study was withdrawn for administrative reason | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 2 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00371696 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 3 | Completed | 01/07/1996 | https://clinicaltrials.gov/study/NCT00000425 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Recruiting | 01/01/2019 | https://clinicaltrials.gov/study/NCT03781544 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 14/03/2017 | https://clinicaltrials.gov/study/NCT02839876 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT01063842 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00635349 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT01728246 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | sinusitis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00377403 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | sinusitis | ACETAMINOPHEN | targetBased | 2 | Completed | 01/04/2017 | https://clinicaltrials.gov/study/NCT03055507 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01075243 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 3 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01920386 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=008c5055-4ae3-498f-a7bf-5bd00f738c6d | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=720366f1-f2d1-6dd6-7bf1-9fdd9378b073 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1f172d58-4050-424b-9743-130d8e618fe8 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 3 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01420653 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d384e88-5c11-4600-ac6c-a38371570138 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 3 | Completed | 30/04/2016 | https://clinicaltrials.gov/study/NCT02735122 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00574015 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00694369 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 2 | Completed | 31/12/2014 | https://clinicaltrials.gov/study/NCT02320708 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42e4219a-a4ef-f090-f6a4-2fd2e213d397 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f5ea858e-76d9-4777-b3dc-5d614f66d485 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07c4cc3d-9a68-42a1-801d-5d4ec107ffad | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 3 | Completed | 19/07/2017 | https://clinicaltrials.gov/study/NCT03224403 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6197eff5-0351-42ef-bb3f-cbc26e6d0d14 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT01082081 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 2 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01960114 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2321b5fb-9b3a-4435-8deb-e8cfd2208b43 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 2 | Completed | 25/04/2019 | https://clinicaltrials.gov/study/NCT04018612 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dad63da3-cd1f-42a8-93d5-44b39aae1268 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 2 | Terminated | 01/08/2016 | https://clinicaltrials.gov/study/NCT02862691 | 0.2 | protect | Difficulty recruiting patients | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dd7b5ba3-652a-443f-b0b9-17abda148e67 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | ACETAMINOPHEN | targetBased | 2 | Withdrawn | https://clinicaltrials.gov/study/NCT01104844 | 0.2 | protect | The study was withdrawn due to administrative reason | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | scoliosis | ACETAMINOPHEN | targetBased | 3 | Completed | 01/06/2021 | https://clinicaltrials.gov/study/NCT04959591 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | scoliosis | ACETAMINOPHEN | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rib fracture | ACETAMINOPHEN | targetBased | 4 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02432456 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rib fracture | ACETAMINOPHEN | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | systemic lupus erythematosus | ACETAMINOPHEN | targetBased | 3 | Recruiting | 26/10/2021 | https://clinicaltrials.gov/study/NCT04963296 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | systemic lupus erythematosus | ACETAMINOPHEN | targetBased | 2 | Completed | 10/05/2005 | https://clinicaltrials.gov/study/NCT00137969 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | systemic lupus erythematosus | ACETAMINOPHEN | targetBased | 3 | Terminated | 22/06/2006 | https://clinicaltrials.gov/study/NCT00381810 | 0.7 | protect | During a safety review of studies U2970g and U2971g, the Data Monitoring Committee recommended that enrollment in this extension trial be terminated. | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | temporomandibular joint disorder | ACETAMINOPHEN | targetBased | 4 | Completed | 16/07/2019 | https://clinicaltrials.gov/study/NCT05529290 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | bone fracture | ACETAMINOPHEN | targetBased | 2 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02521415 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | bone fracture | ACETAMINOPHEN | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02076321 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | bone fracture | ACETAMINOPHEN | targetBased | 4 | Not yet recruiting | 04/11/2022 | https://clinicaltrials.gov/study/NCT05425355 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | sickle cell anemia | ACETAMINOPHEN | targetBased | 4 | Completed | 20/02/2018 | https://clinicaltrials.gov/study/NCT03541980 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rotator cuff tear | ACETAMINOPHEN | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | protect | The study was withdrawn prior to any participants being enrolled. | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rotator cuff tear | ACETAMINOPHEN | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | rotator cuff tear | ACETAMINOPHEN | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | hip fracture | ACETAMINOPHEN | targetBased | 4 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02689024 | 1 | protect | recruitment too slow; intervention was standard care in patients who were not included; acute care pathways changed due to policy regarding hip fracture patients | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | hip fracture | ACETAMINOPHEN | targetBased | 4 | Terminated | 01/12/2016 | https://clinicaltrials.gov/study/NCT02774148 | 1 | protect | Proved difficult to consent patient population due to comorbidities | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | hip fracture | ACETAMINOPHEN | targetBased | 4 | Completed | 21/04/2021 | https://clinicaltrials.gov/study/NCT04837924 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Fabry disease | ACETAMINOPHEN | targetBased | 4 | Recruiting | 10/11/2023 | https://clinicaltrials.gov/study/NCT06019728 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoporosis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00489424 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | muscular disease | ACETAMINOPHEN | targetBased | 4 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT02113566 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 4 | Not yet recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06042426 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 3 | Completed | 01/10/1999 | https://clinicaltrials.gov/study/NCT00568295 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 3 | Completed | 01/05/2000 | https://clinicaltrials.gov/study/NCT00110474 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 2 | Completed | 29/06/2018 | https://clinicaltrials.gov/study/NCT03570554 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01105936 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ACETAMINOPHEN | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00240773 | 0.7 | protect | |||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | avascular necrosis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=50684514-3226-4395-b695-8c69c29becb4 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1f172d58-4050-424b-9743-130d8e618fe8 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d4325b17-23bc-44ac-868d-bb0886853a73 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dd7b5ba3-652a-443f-b0b9-17abda148e67 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02fa9c00-214a-4289-8ab5-d470ce04a70f | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=42e4219a-a4ef-f090-f6a4-2fd2e213d397 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=07c4cc3d-9a68-42a1-801d-5d4ec107ffad | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dad63da3-cd1f-42a8-93d5-44b39aae1268 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=55768b45-fd98-4779-85b6-6a50c628677e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | ACETAMINOPHEN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9d384e88-5c11-4600-ac6c-a38371570138 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | patellofemoral pain syndrome | ACETAMINOPHEN | targetBased | 4 | Unknown status | 01/02/2014 | https://clinicaltrials.gov/study/NCT02241148 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pulpitis | ACETAMINOPHEN | targetBased | 2 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02601911 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | pulpitis | ACETAMINOPHEN | targetBased | 2 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02614118 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Sciatica | ACETAMINOPHEN | targetBased | 4 | Unknown status | 01/03/2016 | https://clinicaltrials.gov/study/NCT02777320 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Sciatica | ACETAMINOPHEN | targetBased | 4 | Unknown status | 01/01/2015 | https://clinicaltrials.gov/study/NCT02504996 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Inguinal hernia | ACETAMINOPHEN | targetBased | 4 | Completed | 08/03/2016 | https://clinicaltrials.gov/study/NCT05919732 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Inguinal hernia | ACETAMINOPHEN | targetBased | 4 | Completed | 08/12/2017 | https://clinicaltrials.gov/study/NCT03558555 | 1 | protect | ||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | muscle cramp | ROPINIROLE | targetBased | 4 | Completed | 02/09/2016 | https://clinicaltrials.gov/study/NCT03176966 | 1 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | fibromyalgia | ROPINIROLE | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00256893 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | systemic lupus erythematosus | PIOGLITAZONE | targetBased | 4 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT01322308 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | PIOGLITAZONE | targetBased | 3 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00811681 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone metastasis | FENTANYL | targetBased | 3 | Unknown status | 01/05/2015 | https://clinicaltrials.gov/study/NCT02426697 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone metastasis | FENTANYL | targetBased | 3 | Unknown status | 01/05/2015 | https://clinicaltrials.gov/study/NCT02426697 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | FENTANYL | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | FENTANYL | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | FENTANYL | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | FENTANYL | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | FENTANYL | targetBased | 4 | Terminated | 10/03/2011 | https://clinicaltrials.gov/study/NCT02056288 | 1 | GoF | protect | Enrollment was suspended due to the duration of the study and poor follow-up response by participants. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | FENTANYL | targetBased | 4 | Terminated | 10/03/2011 | https://clinicaltrials.gov/study/NCT02056288 | 1 | GoF | protect | Enrollment was suspended due to the duration of the study and poor follow-up response by participants. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | FENTANYL | targetBased | 2 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02521415 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | FENTANYL | targetBased | 2 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02521415 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | FENTANYL | targetBased | 4 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02689024 | 1 | GoF | protect | recruitment too slow; intervention was standard care in patients who were not included; acute care pathways changed due to policy regarding hip fracture patients |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | FENTANYL | targetBased | 4 | Terminated | 01/05/2016 | https://clinicaltrials.gov/study/NCT02689024 | 1 | GoF | protect | recruitment too slow; intervention was standard care in patients who were not included; acute care pathways changed due to policy regarding hip fracture patients |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | FENTANYL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00562627 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | FENTANYL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00562627 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | FENTANYL | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT01742897 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | FENTANYL | targetBased | 4 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT01742897 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | FENTANYL | targetBased | 2 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01794533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | FENTANYL | targetBased | 2 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01794533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01774929 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01774929 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 3 | Completed | 01/03/2005 | https://clinicaltrials.gov/study/NCT00228605 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 3 | Completed | 01/03/2005 | https://clinicaltrials.gov/study/NCT00228605 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00524160 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00524160 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00236366 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | FENTANYL | targetBased | 4 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00236366 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 06/01/2021 | https://clinicaltrials.gov/study/NCT04270877 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 06/01/2021 | https://clinicaltrials.gov/study/NCT04270877 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT04502251 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT04502251 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02806440 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02806440 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 4 | Completed | 07/09/2021 | https://clinicaltrials.gov/study/NCT04313972 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 2 | Suspended | 08/10/2020 | https://clinicaltrials.gov/study/NCT04450316 | 0.2 | LoF | protect | Study temporarily suspended awaiting research personnel to resume recruitment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 2 | Suspended | 08/10/2020 | https://clinicaltrials.gov/study/NCT04450316 | 0.2 | LoF | protect | Study temporarily suspended awaiting research personnel to resume recruitment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 2 | Suspended | 08/10/2020 | https://clinicaltrials.gov/study/NCT04450316 | 0.2 | LoF | protect | Study temporarily suspended awaiting research personnel to resume recruitment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 4 | Completed | 07/09/2021 | https://clinicaltrials.gov/study/NCT04313972 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | interstitial cystitis | NALTREXONE | targetBased | 4 | Completed | 07/09/2021 | https://clinicaltrials.gov/study/NCT04313972 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02806440 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT04502251 | 0.2 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | fibromyalgia | NALTREXONE | targetBased | 2 | Completed | 06/01/2021 | https://clinicaltrials.gov/study/NCT04270877 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | MEPERIDINE | targetBased | 3 | Unknown status | 01/12/2004 | https://clinicaltrials.gov/study/NCT00163553 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | MEPERIDINE | targetBased | 3 | Unknown status | 01/12/2004 | https://clinicaltrials.gov/study/NCT00163553 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MEPERIDINE | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | MEPERIDINE | targetBased | 2 | Completed | 18/06/2019 | https://clinicaltrials.gov/study/NCT03968146 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | dental caries | MEPERIDINE | targetBased | 4 | Completed | 25/06/2019 | https://clinicaltrials.gov/study/NCT04068948 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | dental caries | MEPERIDINE | targetBased | 4 | Completed | 25/06/2019 | https://clinicaltrials.gov/study/NCT04068948 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | TRAZODONE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00791739 | 1 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | cardiomyopathy | AMIODARONE | targetBased | 3 | Completed | 01/11/2017 | https://clinicaltrials.gov/study/NCT02924285 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | cardiomyopathy | AMIODARONE | targetBased | 3 | Completed | 01/11/2017 | https://clinicaltrials.gov/study/NCT02924285 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | cardiomyopathy | AMIODARONE | targetBased | 3 | Completed | 01/11/2017 | https://clinicaltrials.gov/study/NCT02924285 | 0.7 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Marfan syndrome | NEBIVOLOL | targetBased | 3 | Unknown status | 01/07/2008 | https://clinicaltrials.gov/study/NCT00683124 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | METHADONE | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01205256 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | METHADONE | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT01205256 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | METHADONE | targetBased | 4 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02206685 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | METHADONE | targetBased | 4 | Completed | 01/05/2014 | https://clinicaltrials.gov/study/NCT02206685 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | juvenile idiopathic scoliosis | METHADONE | targetBased | 4 | Recruiting | 19/10/2022 | https://clinicaltrials.gov/study/NCT05730920 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | juvenile idiopathic scoliosis | METHADONE | targetBased | 4 | Recruiting | 19/10/2022 | https://clinicaltrials.gov/study/NCT05730920 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | METHADONE | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | METHADONE | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | MIRTAZAPINE | targetBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00919295 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ligament rupture | OXYCODONE | targetBased | 4 | Completed | 16/09/2020 | https://clinicaltrials.gov/study/NCT04285853 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ligament rupture | OXYCODONE | targetBased | 4 | Completed | 16/09/2020 | https://clinicaltrials.gov/study/NCT04285853 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | OXYCODONE | targetBased | 4 | Recruiting | 26/02/2019 | https://clinicaltrials.gov/study/NCT03759028 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | OXYCODONE | targetBased | 4 | Recruiting | 26/02/2019 | https://clinicaltrials.gov/study/NCT03759028 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sinusitis | OXYCODONE | targetBased | 2 | Completed | 01/04/2017 | https://clinicaltrials.gov/study/NCT03055507 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sinusitis | OXYCODONE | targetBased | 2 | Completed | 01/04/2017 | https://clinicaltrials.gov/study/NCT03055507 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | OXYCODONE | targetBased | 4 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02464813 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | scoliosis | OXYCODONE | targetBased | 4 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02464813 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spondylolisthesis | OXYCODONE | targetBased | 4 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02464813 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spondylolisthesis | OXYCODONE | targetBased | 4 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02464813 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | joint disease | OXYCODONE | targetBased | 3 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02604446 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | joint disease | OXYCODONE | targetBased | 3 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02604446 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | OXYCODONE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00254631 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | OXYCODONE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00254631 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 4 | Not yet recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06042426 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 4 | Not yet recruiting | 01/09/2023 | https://clinicaltrials.gov/study/NCT06042426 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00979953 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00979953 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 04/12/2012 | https://clinicaltrials.gov/study/NCT01709214 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 04/12/2012 | https://clinicaltrials.gov/study/NCT01709214 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01366014 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01366014 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00784277 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00784277 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 4 | Terminated | 17/01/2008 | https://clinicaltrials.gov/study/NCT00484718 | 1 | GoF | protect | See termination reason in detailed description. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 4 | Terminated | 17/01/2008 | https://clinicaltrials.gov/study/NCT00484718 | 1 | GoF | protect | See termination reason in detailed description. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00902837 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00902837 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Terminated | 30/10/2009 | https://clinicaltrials.gov/study/NCT00985621 | 0.7 | GoF | protect | See termination reason in detailed description. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Terminated | 30/10/2009 | https://clinicaltrials.gov/study/NCT00985621 | 0.7 | GoF | protect | See termination reason in detailed description. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT00312221 | 0.7 | GoF | protect | terminated early for administrative reasons unrelated to safety or efficacy |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | OXYCODONE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT00312221 | 0.7 | GoF | protect | terminated early for administrative reasons unrelated to safety or efficacy |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | OXYCODONE | targetBased | 2 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00941304 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | OXYCODONE | targetBased | 2 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00941304 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | OXYCODONE | targetBased | 2 | Terminated | 01/08/2016 | https://clinicaltrials.gov/study/NCT02862691 | 0.2 | GoF | protect | Difficulty recruiting patients |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | OXYCODONE | targetBased | 2 | Terminated | 01/08/2016 | https://clinicaltrials.gov/study/NCT02862691 | 0.2 | GoF | protect | Difficulty recruiting patients |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Inguinal hernia | OXYCODONE | targetBased | 4 | Unknown status | 01/07/2007 | https://clinicaltrials.gov/study/NCT00480142 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Inguinal hernia | OXYCODONE | targetBased | 4 | Unknown status | 01/07/2007 | https://clinicaltrials.gov/study/NCT00480142 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a78708cd-04d4-4221-a4e4-53680bbe2912 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a78708cd-04d4-4221-a4e4-53680bbe2912 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=92eafd85-0051-44af-ba5c-9194c8deffe2 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=92eafd85-0051-44af-ba5c-9194c8deffe2 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=966ac064-9eb7-4d15-b271-a7880ed1e79a | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=966ac064-9eb7-4d15-b271-a7880ed1e79a | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ESTRADIOL | targetBased | 3 | Completed | 01/02/2004 | https://clinicaltrials.gov/study/NCT00310531 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ESTRADIOL | targetBased | 3 | Completed | 01/02/2004 | https://clinicaltrials.gov/study/NCT00310531 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=966ac064-9eb7-4d15-b271-a7880ed1e79a | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=966ac064-9eb7-4d15-b271-a7880ed1e79a | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12fea717-3ee5-47af-ba43-2c6fd4d85d5b | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=12fea717-3ee5-47af-ba43-2c6fd4d85d5b | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a78708cd-04d4-4221-a4e4-53680bbe2912 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a78708cd-04d4-4221-a4e4-53680bbe2912 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eabc0fb3-da2a-482f-83a7-99b1e823a150 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eabc0fb3-da2a-482f-83a7-99b1e823a150 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac676026-14be-486a-9d64-6702bd9e51ea | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ac676026-14be-486a-9d64-6702bd9e51ea | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=70d00600-b8d3-4820-8db6-40d4536a6f9e | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=70d00600-b8d3-4820-8db6-40d4536a6f9e | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=92eafd85-0051-44af-ba5c-9194c8deffe2 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=92eafd85-0051-44af-ba5c-9194c8deffe2 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09c472c6-a38a-4a3d-b048-6fefd52e2ad3 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09c472c6-a38a-4a3d-b048-6fefd52e2ad3 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | SUFENTANIL | targetBased | 2 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01572116 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | SUFENTANIL | targetBased | 2 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01572116 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 3 | Completed | 09/12/2019 | https://clinicaltrials.gov/study/NCT04172831 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01903265 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 3 | Completed | 01/12/2013 | https://clinicaltrials.gov/study/NCT02015234 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 3 | Completed | 22/07/2020 | https://clinicaltrials.gov/study/NCT04508621 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 3 | Completed | 06/04/2022 | https://clinicaltrials.gov/study/NCT05273749 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | CYCLOBENZAPRINE | targetBased | 3 | Terminated | 01/08/2015 | https://clinicaltrials.gov/study/NCT02589275 | 0.7 | LoF | protect | For business reason unrelated to safety or tolerability,Tonix has discontinued the Fibromyalgia development with TNX102 SL. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0038f081-a867-43ed-8415-9f0003871291 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=06da53af-5b46-4e32-a64c-9677e27ae229 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ec636fad-9537-4001-a691-c7b4cc2b6a96 | 1 | LoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | bone fracture | EPINEPHRINE | targetBased | 3 | Recruiting | 18/04/2024 | https://clinicaltrials.gov/study/NCT06381622 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | shoulder impingement syndrome | EPINEPHRINE | targetBased | 4 | Completed | 01/09/2020 | https://clinicaltrials.gov/study/NCT04594408 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, hip | EPINEPHRINE | targetBased | 4 | Completed | 01/02/2016 | https://clinicaltrials.gov/study/NCT03513276 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, hip | EPINEPHRINE | targetBased | 4 | Completed | 01/06/2015 | https://clinicaltrials.gov/study/NCT02464176 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | stenosing tenosynovitis | EPINEPHRINE | targetBased | 4 | Completed | 01/10/2017 | https://clinicaltrials.gov/study/NCT04023695 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Withdrawn | 01/04/2015 | https://clinicaltrials.gov/study/NCT02365727 | 1 | GoF | protect | Protocol not feasible as written |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02462148 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 3 | Completed | 18/09/2018 | https://clinicaltrials.gov/study/NCT03661996 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 2 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02860949 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00562627 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00901628 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Terminated | 01/10/2015 | https://clinicaltrials.gov/study/NCT02570503 | 1 | GoF | protect | Study's primary aims are no longer clinically impactful, as intrathecal morphine has fallen out of favor and replaced with different agents so that outpatient/23 hr surgery is more predictably achievable. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis, knee | EPINEPHRINE | targetBased | 4 | Withdrawn | 01/02/2016 | https://clinicaltrials.gov/study/NCT02765815 | 1 | GoF | protect | competing protocol |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | rotator cuff tear | EPINEPHRINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis | EPINEPHRINE | targetBased | 4 | Completed | 21/06/2016 | https://clinicaltrials.gov/study/NCT02697955 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis | EPINEPHRINE | targetBased | 4 | Completed | 01/05/2016 | https://clinicaltrials.gov/study/NCT02658149 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | osteoarthritis | EPINEPHRINE | targetBased | 4 | Withdrawn | 30/04/2016 | https://clinicaltrials.gov/study/NCT03119038 | 1 | GoF | protect | Investigator no longer actively pursuing this study. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | dental pulp disease | EPINEPHRINE | targetBased | 4 | Unknown status | 03/01/2018 | https://clinicaltrials.gov/study/NCT03368391 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01706952 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b3d99dc-cb35-4331-96cd-fedb95b96750 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd5ede55-10de-491e-a73f-17a77f89891a | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d785beac-3f68-4df1-926f-34da6773f295 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0babff47-11ed-47b6-a57d-502ca3c86973 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8da22eb1-0965-4d4e-b15d-91db7193548e | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=070d391a-0525-4172-add9-5cb45e0c5d67 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3ed35d7d-616c-43cd-9776-705821afc1f0 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 3 | Unknown status | 01/03/2009 | https://clinicaltrials.gov/study/NCT00852410 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=74b3e639-cd9d-46b0-8505-86b0214cb20b | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=daa651e5-40e1-4e87-9775-3f7cc44dff32 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c07b20c-d222-4b42-87b8-9667705a2945 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f03ce427-2dfa-460c-a1a9-c659d7a35b67 | 1 | GoF | protect | |||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 3 | Completed | 01/06/2014 | https://clinicaltrials.gov/study/NCT02807298 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 2 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01572116 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 2 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01794533 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 4 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01912755 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 2 | Recruiting | 30/04/2023 | https://clinicaltrials.gov/study/NCT05840913 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 4 | Completed | 14/02/2022 | https://clinicaltrials.gov/study/NCT05227300 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 3 | Completed | 01/02/2013 | https://clinicaltrials.gov/study/NCT02226913 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | pulpitis | EPINEPHRINE | targetBased | 4 | Withdrawn | 01/08/2021 | https://clinicaltrials.gov/study/NCT04186299 | 1 | GoF | protect | Study activities never commenced. |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | periodontitis | EPINEPHRINE | targetBased | 4 | Completed | 05/12/2017 | https://clinicaltrials.gov/study/NCT03354312 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | musculoskeletal system disease | EPINEPHRINE | targetBased | 4 | Recruiting | 20/09/2023 | https://clinicaltrials.gov/study/NCT04397484 | 1 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | musculoskeletal system disease | EPINEPHRINE | targetBased | 2 | Recruiting | 27/01/2021 | https://clinicaltrials.gov/study/NCT05355597 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | muscle cramp | EPINEPHRINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=76462d55-5448-0fd0-e053-2991aa0aa49f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | FENTANYL CITRATE | targetBased | 2 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01794533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | FENTANYL CITRATE | targetBased | 2 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01794533 | 0.2 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ETHINYL ESTRADIOL | targetBased | 3 | Withdrawn | 01/07/2005 | https://clinicaltrials.gov/study/NCT00117260 | 0.7 | GoF | protect | Sponsor decision |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ETHINYL ESTRADIOL | targetBased | 3 | Withdrawn | 01/07/2005 | https://clinicaltrials.gov/study/NCT00117260 | 0.7 | GoF | protect | Sponsor decision |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ETHINYL ESTRADIOL | targetBased | 2 | Completed | https://clinicaltrials.gov/study/NCT00320567 | 0.2 | GoF | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ETHINYL ESTRADIOL | targetBased | 2 | Completed | https://clinicaltrials.gov/study/NCT00320567 | 0.2 | GoF | protect | ||
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | DALFAMPRIDINE | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00041717 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01621113 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | proximal spinal muscular atrophy | DALFAMPRIDINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645787 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | spinal cord injury | ALBUTEROL | targetBased | 2 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00755079 | 0.2 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Glycogen storage disease due to acid maltase deficiency | ALBUTEROL | targetBased | 4 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT02405598 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00766350 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT01458964 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | QUETIAPINE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00983320 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Ehlers-Danlos syndrome | CELIPROLOL | targetBased | 3 | Recruiting | 07/11/2022 | https://clinicaltrials.gov/study/NCT05432466 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | dilated cardiomyopathy | CARVEDILOL | targetBased | 3 | Recruiting | 27/04/2023 | https://clinicaltrials.gov/study/NCT05849766 | 0.7 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | dilated cardiomyopathy | CARVEDILOL | targetBased | 4 | Unknown status | 01/01/2006 | https://clinicaltrials.gov/study/NCT00374465 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | idiopathic dilated cardiomyopathy | CARVEDILOL | targetBased | 4 | Completed | 01/09/2000 | https://clinicaltrials.gov/study/NCT01798992 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Duchenne muscular dystrophy | CARVEDILOL | targetBased | 4 | Unknown status | 01/12/2008 | https://clinicaltrials.gov/study/NCT00819845 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Duchenne muscular dystrophy | CARVEDILOL | targetBased | 4 | Unknown status | 01/12/2007 | https://clinicaltrials.gov/study/NCT00606775 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Becker muscular dystrophy | CARVEDILOL | targetBased | 4 | Unknown status | 01/12/2008 | https://clinicaltrials.gov/study/NCT00819845 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | cardiomyopathy | CARVEDILOL | targetBased | 2 | Withdrawn | 01/09/2020 | https://clinicaltrials.gov/study/NCT04190433 | 0.2 | LoF | protect | Administratively closed due to low/no accrual |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | spinal stenosis | KETAMINE | targetBased | 3 | Recruiting | 01/12/2023 | https://clinicaltrials.gov/study/NCT06140927 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | osteoarthritis, knee | KETAMINE | targetBased | 4 | Recruiting | 22/01/2024 | https://clinicaltrials.gov/study/NCT06267638 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | idiopathic scoliosis | KETAMINE | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | dental caries | KETAMINE | targetBased | 4 | Completed | 21/05/2015 | https://clinicaltrials.gov/study/NCT02447289 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | dental caries | KETAMINE | targetBased | 4 | Recruiting | 30/01/2020 | https://clinicaltrials.gov/study/NCT04119180 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | dental caries | KETAMINE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00902395 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | Inguinal hernia | KETAMINE | targetBased | 4 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT02646709 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | rib fracture | KETAMINE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | rib fracture | KETAMINE | targetBased | 3 | Recruiting | 12/06/2021 | https://clinicaltrials.gov/study/NCT04928300 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | rib fracture | KETAMINE | targetBased | 4 | Recruiting | 01/04/2021 | https://clinicaltrials.gov/study/NCT04781673 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | rib fracture | KETAMINE | targetBased | 4 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02432456 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 3 | Completed | 01/01/2016 | https://clinicaltrials.gov/study/NCT02402868 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 4 | Terminated | 09/09/2019 | https://clinicaltrials.gov/study/NCT03781817 | 1 | LoF | protect | Transition/change of primary investigators | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 2 | Completed | 01/12/2015 | https://clinicaltrials.gov/study/NCT02521415 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 4 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00490997 | 1 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 3 | Completed | 05/07/2018 | https://clinicaltrials.gov/study/NCT03525821 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE | targetBased | 3 | Unknown status | 01/10/2017 | https://clinicaltrials.gov/study/NCT02828566 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | KETAMINE | targetBased | 3 | Unknown status | 01/07/2016 | https://clinicaltrials.gov/study/NCT02801292 | 0.7 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | KETAMINE | targetBased | 4 | Terminated | 01/06/2004 | https://clinicaltrials.gov/study/NCT00252122 | 1 | LoF | protect | Recruitment was very slow. | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | KETAMINE | targetBased | 2 | Terminated | 04/03/2008 | https://clinicaltrials.gov/study/NCT00595530 | 0.2 | LoF | protect | Lack of enrollment and patient interest in study | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | KETAMINE | targetBased | 2 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT03296345 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | multiple bone fractures | KETAMINE | targetBased | 2 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01807429 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | temporomandibular joint disorder | KETAMINE | targetBased | 2 | Recruiting | 18/06/2021 | https://clinicaltrials.gov/study/NCT04724759 | 0.2 | LoF | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | lumbar disc herniation | ESMOLOL | targetBased | 2 | Completed | 04/02/2020 | https://clinicaltrials.gov/study/NCT04260685 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | sinusitis | ESMOLOL | targetBased | 2 | Completed | 01/03/2017 | https://clinicaltrials.gov/study/NCT03661346 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | hypertrophic cardiomyopathy | ESMOLOL | targetBased | 4 | Recruiting | 06/10/2021 | https://clinicaltrials.gov/study/NCT05073094 | 1 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | Duchenne muscular dystrophy | TAMOXIFEN CITRATE | targetBased | 3 | Completed | 12/06/2018 | https://clinicaltrials.gov/study/NCT03354039 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | Duchenne muscular dystrophy | TAMOXIFEN CITRATE | targetBased | 3 | Completed | 12/06/2018 | https://clinicaltrials.gov/study/NCT03354039 | 0.7 | protect | ||
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | sickle cell anemia | METHYLPHENIDATE | targetBased | 3 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT01411280 | 0.7 | LoF | protect | |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | sarcoidosis | METHYLPHENIDATE | targetBased | 4 | Completed | 01/06/2006 | https://clinicaltrials.gov/study/NCT00361387 | 1 | LoF | protect | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | systemic lupus erythematosus | MEMANTINE | targetBased | 2 | Recruiting | 23/08/2018 | https://clinicaltrials.gov/study/NCT03527472 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | MEMANTINE | targetBased | 2 | Recruiting | 02/02/2018 | https://clinicaltrials.gov/study/NCT03247218 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | fibromyalgia | MEMANTINE | targetBased | 3 | Unknown status | 01/09/2012 | https://clinicaltrials.gov/study/NCT01653457 | 0.7 | LoF | protect | ||
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | Muscle weakness | GUANIDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 3 | Unknown status | 01/06/2006 | https://clinicaltrials.gov/study/NCT00336167 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | carnitine palmitoyltransferase II deficiency | BEZAFIBRATE | targetBased | 2 | Completed | 01/10/2009 | https://clinicaltrials.gov/study/NCT00983788 | 0.2 | GoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | myelofibrosis | POMALIDOMIDE | targetBased | 2 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00463385 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | systemic scleroderma | POMALIDOMIDE | targetBased | 2 | Terminated | 09/08/2012 | https://clinicaltrials.gov/study/NCT01559129 | 0.2 | LoF | protect | Enrollment was stopped early (see limitations and caveats section). |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | POMALIDOMIDE | targetBased | 2 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01510613 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | POMALIDOMIDE | targetBased | 2 | Recruiting | 23/06/2021 | https://clinicaltrials.gov/study/NCT04895917 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | POMALIDOMIDE | targetBased | 2 | Recruiting | 14/04/2021 | https://clinicaltrials.gov/study/NCT04270175 | 0.2 | LoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteopetrosis | CALCITRIOL | targetBased | 3 | Completed | 01/11/1999 | https://clinicaltrials.gov/study/NCT00004402 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | X-linked hypophosphatemia | CALCITRIOL | targetBased | 4 | Recruiting | 01/01/2017 | https://clinicaltrials.gov/study/NCT03820518 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CALCITRIOL | targetBased | 3 | Completed | 01/09/1997 | https://clinicaltrials.gov/study/NCT00000412 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CALCITRIOL | targetBased | 4 | Unknown status | 01/01/2013 | https://clinicaltrials.gov/study/NCT01765010 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Friedreich ataxia | CALCITRIOL | targetBased | 4 | Completed | 25/08/2021 | https://clinicaltrials.gov/study/NCT04801303 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | CALCITRIOL | targetBased | 2 | Unknown status | 01/04/2013 | https://clinicaltrials.gov/study/NCT01863641 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CALCITRIOL | targetBased | 4 | Completed | 19/06/2011 | https://clinicaltrials.gov/study/NCT01350934 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | renal osteodystrophy | CALCITRIOL | targetBased | 2 | Completed | 01/11/2000 | https://clinicaltrials.gov/study/NCT00560300 | 0.2 | GoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | myelofibrosis | LENALIDOMIDE | targetBased | 2 | Completed | 07/07/2006 | https://clinicaltrials.gov/study/NCT00352794 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | myelofibrosis | LENALIDOMIDE | targetBased | 2 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00087672 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | primary systemic amyloidosis | LENALIDOMIDE | targetBased | 2 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01194791 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | primary systemic amyloidosis | LENALIDOMIDE | targetBased | 2 | Completed | 01/04/2005 | https://clinicaltrials.gov/study/NCT00166413 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | cutaneous lupus erythematosus | LENALIDOMIDE | targetBased | 4 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01408199 | 1 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | LENALIDOMIDE | targetBased | 3 | Terminated | 26/12/2012 | https://clinicaltrials.gov/study/NCT01659658 | 0.7 | LoF | protect | Sponsor's decision |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | LENALIDOMIDE | targetBased | 2 | Completed | 01/02/2008 | https://clinicaltrials.gov/study/NCT00607581 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | LENALIDOMIDE | targetBased | 2 | Active, not recruiting | 25/08/2017 | https://clinicaltrials.gov/study/NCT03252600 | 0.2 | LoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | AL amyloidosis | LENALIDOMIDE | targetBased | 2 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00883623 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 2 | Completed | 01/08/2003 | https://clinicaltrials.gov/study/NCT00238745 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 2 | Completed | 01/08/2003 | https://clinicaltrials.gov/study/NCT00238745 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02090400 | 1 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02090400 | 1 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT02090400 | 1 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE | targetBased | 2 | Completed | 01/08/2003 | https://clinicaltrials.gov/study/NCT00238745 | 0.2 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Glycogen storage disease due to acid maltase deficiency | CLENBUTEROL | targetBased | 2 | Withdrawn | 01/01/2023 | https://clinicaltrials.gov/study/NCT04094948 | 0.2 | GoF | protect | hasn't got the funding |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Kennedy disease | CLENBUTEROL | targetBased | 2 | Recruiting | 13/04/2024 | https://clinicaltrials.gov/study/NCT06169046 | 0.2 | GoF | protect | |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | Friedreich ataxia | BUPROPION | targetBased | 4 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01716221 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | NALBUPHINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | NALBUPHINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | NALBUPHINE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | Hypereosinophilic syndrome | IMATINIB | targetBased | 2 | Terminated | 01/09/2002 | https://clinicaltrials.gov/study/NCT00171860 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | Hypereosinophilic syndrome | IMATINIB | targetBased | 2 | Terminated | 12/06/2003 | https://clinicaltrials.gov/study/NCT00230334 | 0.2 | LoF | protect | terminated by PI due to insufficient accrual |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | Hypereosinophilic syndrome | IMATINIB | targetBased | 2 | Recruiting | 26/09/2002 | https://clinicaltrials.gov/study/NCT00044304 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB | targetBased | 2 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00479934 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB | targetBased | 2 | Completed | 02/01/2008 | https://clinicaltrials.gov/study/NCT00613171 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB | targetBased | 2 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00555581 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB | targetBased | 2 | Unknown status | 01/02/2009 | https://clinicaltrials.gov/study/NCT00573326 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | adult fibrosarcoma | IMATINIB | targetBased | 2 | Completed | 01/05/2004 | https://clinicaltrials.gov/study/NCT00084630 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | localised scleroderma | IMATINIB | targetBased | 2 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00479934 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | fibromyalgia | PRAMIPEXOLE | targetBased | 2 | Terminated | 01/07/2008 | https://clinicaltrials.gov/study/NCT00689052 | 0.2 | GoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | myofascial pain syndrome | CAPSAICIN | targetBased | 2 | Withdrawn | 01/11/2009 | https://clinicaltrials.gov/study/NCT00795106 | 0.2 | protect | Difficulty finding a monitor | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8820b651-cda0-4318-8ef8-903d1a79034e | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis | CAPSAICIN | targetBased | 2 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02558439 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Tendinopathy | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2db23534-31bb-4897-b105-95039166f5a7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Muscle spasm | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2db23534-31bb-4897-b105-95039166f5a7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | lateral epicondylitis | CAPSAICIN | targetBased | 2 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00130949 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Arthralgia | CAPSAICIN | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=M02AB01 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 3 | Completed | 24/01/2018 | https://clinicaltrials.gov/study/NCT03429049 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 3 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00471055 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 3 | Completed | 09/09/2018 | https://clinicaltrials.gov/study/NCT03660943 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 4 | Completed | 01/07/2016 | https://clinicaltrials.gov/study/NCT03124407 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 3 | Completed | 18/09/2018 | https://clinicaltrials.gov/study/NCT03661996 | 0.7 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | CAPSAICIN | targetBased | 2 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00667654 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2db23534-31bb-4897-b105-95039166f5a7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ba11070e-1a36-4d5d-9e48-c37b4e90c037 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aed1e7c1-6197-4152-ae5e-06cf2ec3020f | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=95910cee-0a43-4ac9-aeb9-6a659757b3ba | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b8e3e528-b7cd-e4b7-e053-2995a90a4029 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5dd4fefc-b70a-4850-819c-1cca6f28aa97 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1eced33-f9ee-4757-ab94-75f238f9b2a9 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | arthritis | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d9475b6c-edd1-4ea7-a22c-243e63c4d0f7 | 1 | protect | ||||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | frozen shoulder | CAPSAICIN | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2db23534-31bb-4897-b105-95039166f5a7 | 1 | protect | ||||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | spinal stenosis | OXYMORPHONE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | OXYMORPHONE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | OXYMORPHONE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sinusitis | REMIFENTANIL | targetBased | 4 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01569048 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sinusitis | REMIFENTANIL | targetBased | 4 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01569048 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | REMIFENTANIL | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | REMIFENTANIL | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | REMIFENTANIL | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | REMIFENTANIL | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | REMIFENTANIL | targetBased | 4 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01052324 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | REMIFENTANIL | targetBased | 4 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01052324 | 1 | GoF | protect | |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | MTOR | Serine/threonine-protein kinase mTOR | hypertrophic cardiomyopathy | PERHEXILINE | pathwayBased | 2 | Terminated | 01/08/2016 | https://clinicaltrials.gov/study/NCT02862600 | 0.2 | LoF | protect | Lack of Efficacy |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | MTOR | Serine/threonine-protein kinase mTOR | hypertrophic cardiomyopathy | PERHEXILINE | pathwayBased | 3 | Withdrawn | 01/03/2016 | https://clinicaltrials.gov/study/NCT02431221 | 0.35 | LoF | protect | Lack of efficacy in a preceding study. |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | MTOR | Serine/threonine-protein kinase mTOR | hypertrophic cardiomyopathy | PERHEXILINE | pathwayBased | 2 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00500552 | 0.2 | LoF | protect | |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | MTOR | Serine/threonine-protein kinase mTOR | hypertrophic cardiomyopathy | PERHEXILINE | pathwayBased | 2 | Recruiting | 01/12/2020 | https://clinicaltrials.gov/study/NCT04426578 | 0.2 | LoF | protect | |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | GAA | Lysosomal alpha-glucosidase | Glycogen storage disease due to acid maltase deficiency | DUVOGLUSTAT | targetBased | 2 | Completed | 31/10/2011 | https://clinicaltrials.gov/study/NCT01380743 | 0.2 | GoF | protect | |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | GAA | Lysosomal alpha-glucosidase | Glycogen storage disease due to acid maltase deficiency | DUVOGLUSTAT | targetBased | 2 | Completed | 31/10/2011 | https://clinicaltrials.gov/study/NCT01380743 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | rickets | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00949832 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | CHOLECALCIFEROL | targetBased | 2 | Completed | 10/08/2020 | https://clinicaltrials.gov/study/NCT04768439 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/02/2015 | https://clinicaltrials.gov/study/NCT02186600 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/01/2003 | https://clinicaltrials.gov/study/NCT00160264 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00043719 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | CHOLECALCIFEROL | targetBased | 4 | Completed | 16/12/2020 | https://clinicaltrials.gov/study/NCT05386784 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | juvenile idiopathic arthritis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/03/1995 | https://clinicaltrials.gov/study/NCT00570934 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | CHOLECALCIFEROL | targetBased | 2 | Completed | 21/11/2016 | https://clinicaltrials.gov/study/NCT02786498 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01920568 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | CHOLECALCIFEROL | targetBased | 3 | Unknown status | 01/01/2005 | https://clinicaltrials.gov/study/NCT00133640 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01473602 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01473589 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | hip fracture | CHOLECALCIFEROL | targetBased | 4 | Terminated | 01/06/2013 | https://clinicaltrials.gov/study/NCT01850251 | 1 | GoF | protect | Recruiting problems. |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | hip fracture | CHOLECALCIFEROL | targetBased | 3 | Unknown status | 01/01/2005 | https://clinicaltrials.gov/study/NCT00133640 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteitis deformans | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00103740 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | enamel caries | CHOLECALCIFEROL | targetBased | 4 | Recruiting | 12/12/2023 | https://clinicaltrials.gov/study/NCT06172764 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 24/06/2005 | https://clinicaltrials.gov/study/NCT00112437 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 24/09/2003 | https://clinicaltrials.gov/study/NCT00092066 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00692913 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 02/10/2008 | https://clinicaltrials.gov/study/NCT00729183 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/09/2008 | https://clinicaltrials.gov/study/NCT00757393 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Recruiting | 01/07/2019 | https://clinicaltrials.gov/study/NCT03994172 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d4a71df1-7488-46a2-8ded-dd0db56de618 | 1 | GoF | protect | |||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9f89cb91-bd3b-4103-a8a1-c1c1512fe514 | 1 | GoF | protect | |||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c0ec7e75-03b9-4b5b-ca8b-58cb0f65b5d9 | 1 | GoF | protect | |||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Recruiting | 12/10/2017 | https://clinicaltrials.gov/study/NCT03183557 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Terminated | 01/10/2009 | https://clinicaltrials.gov/study/NCT00960934 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 03/12/2008 | https://clinicaltrials.gov/study/NCT00752557 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/10/1992 | https://clinicaltrials.gov/study/NCT00357643 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Unknown status | 19/01/2017 | https://clinicaltrials.gov/study/NCT03040531 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Unknown status | 01/09/2005 | https://clinicaltrials.gov/study/NCT00668941 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/12/2012 | https://clinicaltrials.gov/study/NCT01904110 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/05/2000 | https://clinicaltrials.gov/study/NCT00352170 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Terminated | 01/03/2003 | https://clinicaltrials.gov/study/NCT00058188 | 0.7 | GoF | protect | Closed by the research committee |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Unknown status | 01/06/2011 | https://clinicaltrials.gov/study/NCT02518763 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 09/06/2010 | https://clinicaltrials.gov/study/NCT01120600 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00022087 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/07/2002 | https://clinicaltrials.gov/study/NCT00043719 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT00996801 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/05/2000 | https://clinicaltrials.gov/study/NCT00138983 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/02/2007 | https://clinicaltrials.gov/study/NCT00421343 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00043069 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 04/07/2011 | https://clinicaltrials.gov/study/NCT01675297 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 13/04/2009 | https://clinicaltrials.gov/study/NCT00885170 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Unknown status | 01/01/2013 | https://clinicaltrials.gov/study/NCT01765010 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00092079 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteogenesis imperfecta | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT01713231 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | CHOLECALCIFEROL | targetBased | 3 | Completed | 03/10/2021 | https://clinicaltrials.gov/study/NCT05326841 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | CHOLECALCIFEROL | targetBased | 2 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00710021 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | CHOLECALCIFEROL | targetBased | 2 | Completed | 01/03/2020 | https://clinicaltrials.gov/study/NCT05430087 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | CHOLECALCIFEROL | targetBased | 2 | Terminated | 01/06/2013 | https://clinicaltrials.gov/study/NCT01709474 | 0.2 | GoF | protect | Due to slow enrollment |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT00996801 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 03/12/2007 | https://clinicaltrials.gov/study/NCT00620113 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 19/06/2011 | https://clinicaltrials.gov/study/NCT01350934 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00271713 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 2 | Completed | 05/02/2020 | https://clinicaltrials.gov/study/NCT04321837 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 20/03/2008 | https://clinicaltrials.gov/study/NCT00729651 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Completed | 01/08/1996 | https://clinicaltrials.gov/study/NCT00670501 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 4 | Completed | 12/07/2012 | https://clinicaltrials.gov/study/NCT04609189 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Withdrawn | 01/05/2012 | https://clinicaltrials.gov/study/NCT01552122 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | CHOLECALCIFEROL | targetBased | 3 | Terminated | 13/09/2007 | https://clinicaltrials.gov/study/NCT00529373 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoarthritis, knee | CHOLECALCIFEROL | targetBased | 4 | Recruiting | 07/07/2021 | https://clinicaltrials.gov/study/NCT04739592 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoarthritis, knee | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00599807 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoarthritis, knee | CHOLECALCIFEROL | targetBased | 2 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00306774 | 0.2 | GoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Arthralgia | ZUCAPSAICIN | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=M02AB02 | 0.7 | GoF | protect | |||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ZUCAPSAICIN | targetBased | 3 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00995306 | 0.7 | GoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | osteoarthritis, knee | ZUCAPSAICIN | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00077935 | 0.7 | GoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | peripheral primitive neuroectodermal tumor of bone | VINCRISTINE | targetBased | 3 | Active, not recruiting | 22/11/2010 | https://clinicaltrials.gov/study/NCT01231906 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | Ewing sarcoma of bone | VINCRISTINE | targetBased | 2 | Completed | 01/02/2008 | https://clinicaltrials.gov/study/NCT00516295 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINCRISTINE | targetBased | 3 | Recruiting | 14/09/2021 | https://clinicaltrials.gov/study/NCT04994132 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINCRISTINE | targetBased | 3 | Active, not recruiting | 01/06/2016 | https://clinicaltrials.gov/study/NCT02567435 | 0.7 | LoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | RALOXIFENE HYDROCHLORIDE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/evista | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | RALOXIFENE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fcaaa6dc-74e8-4fb8-800c-5574bf0f8de9 | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/05/2003 | https://clinicaltrials.gov/study/NCT00163137 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | LASOFOXIFENE | targetBased | 2 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00143273 | 0.2 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/11/2001 | https://clinicaltrials.gov/study/NCT00141323 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/05/2003 | https://clinicaltrials.gov/study/NCT00163137 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/05/2003 | https://clinicaltrials.gov/study/NCT00163137 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 2 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00143273 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 2 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00143273 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/11/2001 | https://clinicaltrials.gov/study/NCT00141323 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | LASOFOXIFENE | targetBased | 3 | Completed | 01/11/2001 | https://clinicaltrials.gov/study/NCT00141323 | 0.7 | protect | ||
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | ALOX12 | Polyunsaturated fatty acid lipoxygenase ALOX12 | rheumatic disease | BENOXAPROFEN | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=M01AE06 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | Inguinal hernia | ISOFLURANE | targetBased | 3 | Withdrawn | 01/10/2015 | https://clinicaltrials.gov/study/NCT02521831 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | Inguinal hernia | ISOFLURANE | targetBased | 3 | Withdrawn | 01/10/2015 | https://clinicaltrials.gov/study/NCT02521831 | 0.7 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | Friedreich ataxia | LERIGLITAZONE | targetBased | 2 | Completed | 26/03/2019 | https://clinicaltrials.gov/study/NCT03917225 | 0.2 | GoF | protect | |
| Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer (HTRF) Assay | CACNA1B_modulators | CACNA1B | Voltage-dependent N-type calcium channel subunit alpha-1D | sickle cell anemia | LEVETIRACETAM | targetBased | 2 | Terminated | 23/06/2008 | https://clinicaltrials.gov/study/NCT00489281 | 0.2 | LoF | protect | Initiation of CMS BMT study for sickle-cell disease operating under NCT01166009 made further accrual to this study impossible. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | fibromyalgia | ROTIGOTINE | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00464737 | 0.2 | GoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy | AMIFAMPRIDINE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | McCune-Albright syndrome | FULVESTRANT | targetBased | 2 | Completed | 31/01/2006 | https://clinicaltrials.gov/study/NCT00278915 | 0.2 | LoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | systemic lupus erythematosus | FULVESTRANT | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00417430 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | McCune-Albright syndrome | FULVESTRANT | targetBased | 2 | Completed | 31/01/2006 | https://clinicaltrials.gov/study/NCT00278915 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | McCune-Albright syndrome | FULVESTRANT | targetBased | 2 | Completed | 31/01/2006 | https://clinicaltrials.gov/study/NCT00278915 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | FULVESTRANT | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00417430 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | FULVESTRANT | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00417430 | 0.2 | LoF | protect | |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | osteoarthritis | MODAFINIL | targetBased | 4 | Completed | 01/07/2014 | https://clinicaltrials.gov/study/NCT02155257 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | fibromyalgia | PSILOCYBINE | targetBased | 2 | Active, not recruiting | 27/09/2023 | https://clinicaltrials.gov/study/NCT05128162 | 0.2 | GoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | toothache | SB-705498 | targetBased | 2 | Completed | 07/12/2005 | https://clinicaltrials.gov/study/NCT00281684 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | fibrodysplasia ossificans progressiva | SARACATINIB | targetBased | 2 | Recruiting | 05/08/2020 | https://clinicaltrials.gov/study/NCT04307953 | 0.2 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | muscle cramp | NIMODIPINE | targetBased | 2 | Completed | 24/11/2021 | https://clinicaltrials.gov/study/NCT05131867 | 0.2 | LoF | protect | |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | spondylolisthesis | DESMOPRESSIN | targetBased | 4 | Not yet recruiting | 28/03/2024 | https://clinicaltrials.gov/study/NCT06255366 | 1 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myositis | TOFACITINIB | targetBased | 2 | Recruiting | 10/06/2022 | https://clinicaltrials.gov/study/NCT05400889 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 2 | Recruiting | 26/04/2023 | https://clinicaltrials.gov/study/NCT05814939 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 4 | Completed | 01/01/2022 | https://clinicaltrials.gov/study/NCT06310057 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 4 | Unknown status | 16/01/2018 | https://clinicaltrials.gov/study/NCT03504072 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 2 | Completed | 01/04/2013 | https://clinicaltrials.gov/study/NCT01786668 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 3 | Completed | 07/06/2018 | https://clinicaltrials.gov/study/NCT03502616 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 2 | Unknown status | 01/09/2017 | https://clinicaltrials.gov/study/NCT03738956 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB | targetBased | 4 | Not yet recruiting | 10/11/2023 | https://clinicaltrials.gov/study/NCT06112665 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic scleroderma | TOFACITINIB | targetBased | 2 | Recruiting | 01/11/2023 | https://clinicaltrials.gov/study/NCT06044844 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | dermatomyositis | TOFACITINIB | targetBased | 4 | Enrolling by invitation | 19/03/2024 | https://clinicaltrials.gov/study/NCT06438679 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | polymyalgia rheumatica | TOFACITINIB | targetBased | 2 | Completed | 01/01/2021 | https://clinicaltrials.gov/study/NCT04799262 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | polymyalgia rheumatica | TOFACITINIB | targetBased | 3 | Not yet recruiting | 15/01/2024 | https://clinicaltrials.gov/study/NCT06172361 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=68e3d6b2-7838-4d2d-a417-09d919b43e13 | 1 | LoF | protect | |||
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Completed | 15/09/2017 | https://clinicaltrials.gov/study/NCT03736161 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 2 | Not yet recruiting | 05/02/2024 | https://clinicaltrials.gov/study/NCT06176508 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 4 | Recruiting | 15/11/2021 | https://clinicaltrials.gov/study/NCT05080218 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Completed | 20/01/2014 | https://clinicaltrials.gov/study/NCT01877668 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Completed | 10/08/2018 | https://clinicaltrials.gov/study/NCT03486457 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Recruiting | 19/10/2020 | https://clinicaltrials.gov/study/NCT04610476 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Completed | 17/02/2014 | https://clinicaltrials.gov/study/NCT01976364 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01882439 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | TOFACITINIB | targetBased | 2 | Active, not recruiting | 18/03/2013 | https://clinicaltrials.gov/study/NCT01500551 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | TOFACITINIB | targetBased | 3 | Completed | 10/05/2018 | https://clinicaltrials.gov/study/NCT03000439 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | TOFACITINIB | targetBased | 3 | Completed | 10/06/2016 | https://clinicaltrials.gov/study/NCT02592434 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myocarditis | TOFACITINIB | targetBased | 2 | Not yet recruiting | 01/07/2024 | https://clinicaltrials.gov/study/NCT06393972 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00085956 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00085956 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00088010 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00088010 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00383422 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00383422 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00383422 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/04/2004 | https://clinicaltrials.gov/study/NCT00085956 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ARZOXIFENE | targetBased | 3 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00088010 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ARZOXIFENE | targetBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00767299 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ARZOXIFENE | targetBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00767299 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ARZOXIFENE | targetBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00767299 | 0.2 | protect | ||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rib fracture | HYDROCODONE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Terminated | 07/06/2021 | https://clinicaltrials.gov/study/NCT04905563 | 1 | GoF | protect | Clinical evidence supporting the hypothesis of this study has been published |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Terminated | 07/06/2021 | https://clinicaltrials.gov/study/NCT04905563 | 1 | GoF | protect | Clinical evidence supporting the hypothesis of this study has been published |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 15/04/2019 | https://clinicaltrials.gov/study/NCT06187584 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 15/04/2019 | https://clinicaltrials.gov/study/NCT06187584 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 12/09/2023 | https://clinicaltrials.gov/study/NCT05640674 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 12/09/2023 | https://clinicaltrials.gov/study/NCT05640674 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 3 | Completed | 01/02/2006 | https://clinicaltrials.gov/study/NCT00298974 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Terminated | 07/06/2021 | https://clinicaltrials.gov/study/NCT04905563 | 1 | GoF | protect | Clinical evidence supporting the hypothesis of this study has been published |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 15/04/2019 | https://clinicaltrials.gov/study/NCT06187584 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | humerus fracture | HYDROCODONE | targetBased | 4 | Enrolling by invitation | 12/09/2023 | https://clinicaltrials.gov/study/NCT05640674 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | toothache | HYDROCODONE | targetBased | 4 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00574015 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | HYDROCODONE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | HYDROCODONE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 3 | Completed | 01/02/2006 | https://clinicaltrials.gov/study/NCT00298974 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE | targetBased | 3 | Completed | 01/02/2006 | https://clinicaltrials.gov/study/NCT00298974 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | HYDROCODONE | targetBased | 4 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00574015 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | HYDROCODONE | targetBased | 4 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00574015 | 1 | GoF | protect | |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Withdrawn | 01/12/2021 | https://clinicaltrials.gov/study/NCT04427540 | 0.2 | GoF | protect | Study was not initiated; no subjects were consented or enrolled |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Withdrawn | 01/01/2024 | https://clinicaltrials.gov/study/NCT04493229 | 0.2 | GoF | protect | study team decided not to proceed with project |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Not yet recruiting | 01/06/2025 | https://clinicaltrials.gov/study/NCT04435704 | 0.2 | GoF | protect | |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Withdrawn | 01/12/2023 | https://clinicaltrials.gov/study/NCT04431193 | 0.2 | GoF | protect | Study will be replaced by a revised study protocol |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Recruiting | 04/11/2020 | https://clinicaltrials.gov/study/NCT04431206 | 0.2 | GoF | protect | |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Recruiting | 06/08/2019 | https://clinicaltrials.gov/study/NCT03878589 | 0.2 | GoF | protect | |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis, knee | OXYTOCIN | targetBased | 2 | Withdrawn | 01/01/2023 | https://clinicaltrials.gov/study/NCT04429880 | 0.2 | GoF | protect | Study was not initiated; no subjects enrolled. A replacement study will be submitted |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | osteoarthritis | OXYTOCIN | targetBased | 2 | Recruiting | 06/08/2019 | https://clinicaltrials.gov/study/NCT03878589 | 0.2 | GoF | protect | |
| Fluorescence-based primary cell-based high throughput screening assay to identify agonists of the Oxytocin Receptor (OXTR). | OXTR | OXTR | Oxytocin receptor | interstitial cystitis | OXYTOCIN | targetBased | 4 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT00919802 | 1 | GoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | muscle cramp | NICARDIPINE | targetBased | 3 | Completed | 01/11/2020 | https://clinicaltrials.gov/study/NCT04538534 | 0.7 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | spinal stenosis | NICARDIPINE | targetBased | 4 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT02271191 | 1 | LoF | protect | |
| uHTS identification of DNMT1 inhibitors in a Fluorescent Molecular Beacon assay | DNMT1 | DNMT1 | DNA methyltransferase-1 | myelofibrosis | AZACITIDINE | targetBased | 2 | Completed | 01/06/2005 | https://clinicaltrials.gov/study/NCT00569660 | 0.2 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | hypertrophic cardiomyopathy | AMLODIPINE | targetBased | 2 | Completed | 08/01/2020 | https://clinicaltrials.gov/study/NCT04164732 | 0.2 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | osteoarthritis | AMLODIPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=322f926c-99ac-49f8-9cc5-584594f2891f | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00411164 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00411164 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | HYDROMORPHONE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rib fracture | HYDROMORPHONE | targetBased | 4 | Unknown status | 06/11/2018 | https://clinicaltrials.gov/study/NCT03619785 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00411164 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00411164 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE | targetBased | 3 | Terminated | 13/08/2019 | https://clinicaltrials.gov/study/NCT03933397 | 0.7 | GoF | protect | Due to COVID enrollment numbers needed to meet the primary endpoint will not be met. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE | targetBased | 3 | Terminated | 13/08/2019 | https://clinicaltrials.gov/study/NCT03933397 | 0.7 | GoF | protect | Due to COVID enrollment numbers needed to meet the primary endpoint will not be met. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL VALERATE | targetBased | 4 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT00860964 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRADIOL VALERATE | targetBased | 4 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT00860964 | 1 | GoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | NILOTINIB | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01166139 | 0.2 | LoF | protect | |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of myeloid cell leukemia sequence 1 (MCL1) interactions with BIM-BH3 peptide. | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| uHTS of Mcl-1/Bid interaction inhibitors | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| uHTS of Mcl-1/Noxa interaction inhibitors | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Multiplexed high-throughput screen for small molecule regulators of Bcl-2 family protein interactions, specifically Bim-Bfl-1 | BCL2A1_modulators | BCL2A1 | Bcl-2-related protein A1 | myelofibrosis | OBATOCLAX | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Primary biochemical high throughput screening assay to identify inhibitors of BCL2-related protein, long isoform (BCLXL). | BCL2L1_modulators | BCL2L1 | Bcl-2-like protein 1 | myelofibrosis | OBATOCLAX | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 4 | Recruiting | 31/12/2022 | https://clinicaltrials.gov/study/NCT05666336 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 2 | Active, not recruiting | 28/12/2017 | https://clinicaltrials.gov/study/NCT03030118 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 4 | Completed | 05/06/2017 | https://clinicaltrials.gov/study/NCT03122431 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 4 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00413361 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 2 | Terminated | 20/11/2013 | https://clinicaltrials.gov/study/NCT01946880 | 0.2 | LoF | protect | Slow enrollment. |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01551069 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | cutaneous lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01551069 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | cutaneous lupus erythematosus | HYDROXYCHLOROQUINE | targetBased | 4 | Completed | 05/06/2017 | https://clinicaltrials.gov/study/NCT03122431 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | ankylosing spondylitis | HYDROXYCHLOROQUINE | targetBased | 4 | Not yet recruiting | 07/10/2019 | https://clinicaltrials.gov/study/NCT04077957 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | osteoarthritis | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148043 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | sarcoidosis | HYDROXYCHLOROQUINE | targetBased | 4 | Not yet recruiting | 01/04/2024 | https://clinicaltrials.gov/study/NCT05841758 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | osteoarthritis, knee | HYDROXYCHLOROQUINE | targetBased | 2 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01645176 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | pulmonary sarcoidosis | HYDROXYCHLOROQUINE | targetBased | 3 | Not yet recruiting | 01/03/2022 | https://clinicaltrials.gov/study/NCT05247554 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | pulmonary sarcoidosis | HYDROXYCHLOROQUINE | targetBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT02200146 | 0.7 | LoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | ERGOCALCIFEROL | targetBased | 2 | Completed | 10/08/2020 | https://clinicaltrials.gov/study/NCT04768439 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | rickets | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/01/2004 | https://clinicaltrials.gov/study/NCT00949832 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | rickets | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT01578434 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | Osteopenia | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/09/2007 | https://clinicaltrials.gov/study/NCT00463268 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | chronic rhinosinusitis with nasal polyps | ERGOCALCIFEROL | targetBased | 3 | Unknown status | 01/05/2016 | https://clinicaltrials.gov/study/NCT02668861 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | sickle cell anemia | ERGOCALCIFEROL | targetBased | 2 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT01331148 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | sickle cell anemia | ERGOCALCIFEROL | targetBased | 3 | Completed | 30/11/2018 | https://clinicaltrials.gov/study/NCT03417947 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | bone fracture | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02753283 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | hip fracture | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT00424619 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | hip fracture | ERGOCALCIFEROL | targetBased | 3 | Unknown status | 17/05/2017 | https://clinicaltrials.gov/study/NCT03133195 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | hypophosphatemic rickets | ERGOCALCIFEROL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=00c063ce-5a2c-4ccb-87df-6ec3df0c9fa6 | 1 | GoF | protect | |||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteitis deformans | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/01/2001 | https://clinicaltrials.gov/study/NCT00051636 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteitis deformans | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00103740 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | cutaneous lupus erythematosus | ERGOCALCIFEROL | targetBased | 2 | Terminated | 01/12/2011 | https://clinicaltrials.gov/study/NCT01498406 | 0.2 | GoF | protect | secondary to funding issues and low enrollment |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteogenesis imperfecta type 3 | ERGOCALCIFEROL | targetBased | 2 | Completed | 11/09/2017 | https://clinicaltrials.gov/study/NCT03118570 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | X-linked hypophosphatemia | ERGOCALCIFEROL | targetBased | 3 | Completed | 08/09/2016 | https://clinicaltrials.gov/study/NCT02915705 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | enamel caries | ERGOCALCIFEROL | targetBased | 4 | Recruiting | 12/12/2023 | https://clinicaltrials.gov/study/NCT06172764 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/04/2002 | https://clinicaltrials.gov/study/NCT00048061 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Unknown status | 01/01/2007 | https://clinicaltrials.gov/study/NCT00634686 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00000611 | 0.7 | GoF | protect | ||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00887354 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 2 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00663221 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00329797 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00404820 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Active, not recruiting | 29/04/2017 | https://clinicaltrials.gov/study/NCT03293108 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02753283 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 2 | Completed | 03/12/2008 | https://clinicaltrials.gov/study/NCT00752557 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01753856 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/10/2011 | https://clinicaltrials.gov/study/NCT01656512 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 2 | Recruiting | 12/10/2017 | https://clinicaltrials.gov/study/NCT03186131 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/04/2001 | https://clinicaltrials.gov/study/NCT02416271 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/03/2003 | https://clinicaltrials.gov/study/NCT00054418 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Recruiting | 09/10/2021 | https://clinicaltrials.gov/study/NCT05203588 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteogenesis imperfecta | ERGOCALCIFEROL | targetBased | 2 | Completed | 11/09/2017 | https://clinicaltrials.gov/study/NCT03118570 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteogenesis imperfecta type 4 | ERGOCALCIFEROL | targetBased | 2 | Completed | 11/09/2017 | https://clinicaltrials.gov/study/NCT03118570 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT00092014 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Active, not recruiting | 31/03/2022 | https://clinicaltrials.gov/study/NCT05338086 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/06/2016 | https://clinicaltrials.gov/study/NCT02753283 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02014467 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Unknown status | 01/06/2017 | https://clinicaltrials.gov/study/NCT03158246 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/08/1996 | https://clinicaltrials.gov/study/NCT00670501 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Recruiting | 03/01/2023 | https://clinicaltrials.gov/study/NCT05630768 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00271713 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01709110 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | spinal cord injury | ERGOCALCIFEROL | targetBased | 2 | Active, not recruiting | 01/10/2021 | https://clinicaltrials.gov/study/NCT05008484 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | femoral neck fracture | ERGOCALCIFEROL | targetBased | 3 | Terminated | 01/09/2014 | https://clinicaltrials.gov/study/NCT01908751 | 0.7 | GoF | protect | Continuing beyond the pilot phase of the trial was deemed unfeasible |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoarthritis, knee | ERGOCALCIFEROL | targetBased | 4 | Completed | 01/02/2020 | https://clinicaltrials.gov/study/NCT04177758 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoarthritis, knee | ERGOCALCIFEROL | targetBased | 3 | Completed | 01/08/2010 | https://clinicaltrials.gov/study/NCT01176344 | 0.7 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | ATROPINE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | ATROPINE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | ATROPINE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | ATROPINE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | ATROPINE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | BUPRENORPHINE | targetBased | 2 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT03380533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Intervertebral disk degeneration | BUPRENORPHINE | targetBased | 3 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT01476774 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Intervertebral disk degeneration | BUPRENORPHINE | targetBased | 3 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT01476774 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00313846 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/11/1996 | https://clinicaltrials.gov/study/NCT00315848 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/12/2003 | https://clinicaltrials.gov/study/NCT00315458 | 0.7 | GoF | protect | Administrative reasons. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/1999 | https://clinicaltrials.gov/study/NCT00314652 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT01141283 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01298765 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT01135524 | 0.7 | GoF | protect | This study was terminated early for administrative reasons. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/01/2004 | https://clinicaltrials.gov/study/NCT00320801 | 0.7 | GoF | protect | This study was terminated early due to administrative reasons. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00312572 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT00312221 | 0.7 | GoF | protect | terminated early for administrative reasons unrelated to safety or efficacy |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01019265 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | toothache | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00941304 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Intervertebral disk degeneration | BUPRENORPHINE | targetBased | 3 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT01476774 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | BUPRENORPHINE | targetBased | 2 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT03380533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | BUPRENORPHINE | targetBased | 2 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT03380533 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | sickle cell anemia | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT03492099 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00313846 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00313846 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT01135524 | 0.7 | GoF | protect | This study was terminated early for administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT01135524 | 0.7 | GoF | protect | This study was terminated early for administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT01141283 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT01141283 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/12/2003 | https://clinicaltrials.gov/study/NCT00315458 | 0.7 | GoF | protect | Administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/12/2003 | https://clinicaltrials.gov/study/NCT00315458 | 0.7 | GoF | protect | Administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00312572 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00312572 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01298765 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01298765 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01019265 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01019265 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT00312221 | 0.7 | GoF | protect | terminated early for administrative reasons unrelated to safety or efficacy |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/04/2004 | https://clinicaltrials.gov/study/NCT00312221 | 0.7 | GoF | protect | terminated early for administrative reasons unrelated to safety or efficacy |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT00324038 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/11/1996 | https://clinicaltrials.gov/study/NCT00315848 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/11/1996 | https://clinicaltrials.gov/study/NCT00315848 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/01/2004 | https://clinicaltrials.gov/study/NCT00320801 | 0.7 | GoF | protect | This study was terminated early due to administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Terminated | 01/01/2004 | https://clinicaltrials.gov/study/NCT00320801 | 0.7 | GoF | protect | This study was terminated early due to administrative reasons. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/1999 | https://clinicaltrials.gov/study/NCT00314652 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | BUPRENORPHINE | targetBased | 3 | Completed | 01/06/1999 | https://clinicaltrials.gov/study/NCT00314652 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT03492099 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2018 | https://clinicaltrials.gov/study/NCT03492099 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00941304 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | BUPRENORPHINE | targetBased | 2 | Completed | 01/08/2009 | https://clinicaltrials.gov/study/NCT00941304 | 0.2 | GoF | protect | |
| Total Fluorescence Counterscreen for Inhibitors of the Interaction of Thyroid Hormone Receptor and Steroid Receptor Coregulator 2 | THRB | THRB | Thyroid hormone receptor beta | systemic lupus erythematosus | LEVOTHYROXINE | targetBased | 4 | Withdrawn | 01/01/2011 | https://clinicaltrials.gov/study/NCT01276782 | 1 | GoF | protect | Withdraw the study because further analysis showed that it would be futile. |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | GAA | Lysosomal alpha-glucosidase | Glycogen storage disease due to acid maltase deficiency | DUVOGLUSTAT HYDROCHLORIDE | targetBased | 2 | Completed | 31/10/2011 | https://clinicaltrials.gov/study/NCT01380743 | 0.2 | GoF | protect | |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | GAA | Lysosomal alpha-glucosidase | Glycogen storage disease due to acid maltase deficiency | DUVOGLUSTAT HYDROCHLORIDE | targetBased | 2 | Completed | 31/10/2011 | https://clinicaltrials.gov/study/NCT01380743 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | DIACETYLMORPHINE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | DIACETYLMORPHINE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | Hypereosinophilic syndrome | IMATINIB MESYLATE | targetBased | 2 | Terminated | 12/06/2003 | https://clinicaltrials.gov/study/NCT00230334 | 0.2 | LoF | protect | terminated by PI due to insufficient accrual |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | Hypereosinophilic syndrome | IMATINIB MESYLATE | targetBased | 2 | Terminated | 01/09/2002 | https://clinicaltrials.gov/study/NCT00171860 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB MESYLATE | targetBased | 2 | Completed | 02/01/2008 | https://clinicaltrials.gov/study/NCT00613171 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB MESYLATE | targetBased | 2 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00555581 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | IMATINIB MESYLATE | targetBased | 2 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00479934 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | adult fibrosarcoma | IMATINIB MESYLATE | targetBased | 2 | Completed | 01/05/2004 | https://clinicaltrials.gov/study/NCT00084630 | 0.2 | LoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | localised scleroderma | IMATINIB MESYLATE | targetBased | 2 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00479934 | 0.2 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRONE SULFURIC ACID | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdbf5b-b572-448f-80e9-b3e812959901 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTRONE SULFURIC ACID | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdbf5b-b572-448f-80e9-b3e812959901 | 1 | GoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | peripheral primitive neuroectodermal tumor of bone | VINCRISTINE SULFATE | targetBased | 3 | Active, not recruiting | 22/11/2010 | https://clinicaltrials.gov/study/NCT01231906 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | Ewing sarcoma of bone | VINCRISTINE SULFATE | targetBased | 2 | Completed | 01/02/2008 | https://clinicaltrials.gov/study/NCT00516295 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINCRISTINE SULFATE | targetBased | 3 | Recruiting | 14/09/2021 | https://clinicaltrials.gov/study/NCT04994132 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINCRISTINE SULFATE | targetBased | 3 | Active, not recruiting | 01/06/2016 | https://clinicaltrials.gov/study/NCT02567435 | 0.7 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE | targetBased | 3 | Withdrawn | 01/03/2011 | https://clinicaltrials.gov/study/NCT01214291 | 0.7 | protect | cost of conducting the study and increased burden on the clinical trial professionals make it impossible for us to proceed with the development of the drug. | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE | targetBased | 3 | Withdrawn | 01/03/2011 | https://clinicaltrials.gov/study/NCT01214291 | 0.7 | protect | cost of conducting the study and increased burden on the clinical trial professionals make it impossible for us to proceed with the development of the drug. | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | bone fracture | TOREMIFENE | targetBased | 3 | Withdrawn | 01/03/2011 | https://clinicaltrials.gov/study/NCT01214291 | 0.7 | protect | cost of conducting the study and increased burden on the clinical trial professionals make it impossible for us to proceed with the development of the drug. | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | bone fracture | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | TOREMIFENE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | radius fracture | TERIPARATIDE | targetBased | 2 | Completed | 01/12/2004 | https://clinicaltrials.gov/study/NCT00190944 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/11/2003 | https://clinicaltrials.gov/study/NCT00532207 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01750086 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Unknown status | 01/06/2013 | https://clinicaltrials.gov/study/NCT01945788 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/08/2003 | https://clinicaltrials.gov/study/NCT00542984 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00079924 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00239629 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/08/2014 | https://clinicaltrials.gov/study/NCT02176382 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 02/09/2019 | https://clinicaltrials.gov/study/NCT04026256 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00532545 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01321723 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/10/2001 | https://clinicaltrials.gov/study/NCT00035256 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00557310 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00927186 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2002 | https://clinicaltrials.gov/study/NCT00543218 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 31/01/2013 | https://clinicaltrials.gov/study/NCT01796301 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01343004 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/10/2012 | https://clinicaltrials.gov/study/NCT01709110 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00046137 | 0.7 | GoF | protect | ||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/teriparatide-sun | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/06/2003 | https://clinicaltrials.gov/study/NCT00543023 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/08/1996 | https://clinicaltrials.gov/study/NCT00670501 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/08/2002 | https://clinicaltrials.gov/study/NCT00191425 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | hip fracture | TERIPARATIDE | targetBased | 3 | Unknown status | 17/05/2017 | https://clinicaltrials.gov/study/NCT03133195 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01360424 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01343004 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00439244 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/11/2005 | https://clinicaltrials.gov/study/NCT00259298 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00051558 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2003 | https://clinicaltrials.gov/study/NCT00065637 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00577863 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/04/2009 | https://clinicaltrials.gov/study/NCT00887354 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00535860 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1b007339-dd0d-f019-5e0a-9b1b0f75011c | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/07/2008 | https://clinicaltrials.gov/study/NCT01153425 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2016 | https://clinicaltrials.gov/study/NCT03002428 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/03/2006 | https://clinicaltrials.gov/study/NCT01535027 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00191893 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Recruiting | 01/07/2019 | https://clinicaltrials.gov/study/NCT03994172 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/01/2007 | https://clinicaltrials.gov/study/NCT00433160 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/02/2005 | https://clinicaltrials.gov/study/NCT00191867 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01430104 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/01/2011 | https://clinicaltrials.gov/study/NCT01293292 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/06/2009 | https://clinicaltrials.gov/study/NCT00926380 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT00503399 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/teriparatide-sun | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 02/08/2012 | https://clinicaltrials.gov/study/NCT01440803 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01011556 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/04/2001 | https://clinicaltrials.gov/study/NCT02416271 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00005006 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Unknown status | 01/05/2014 | https://clinicaltrials.gov/study/NCT02156960 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Recruiting | 21/03/2022 | https://clinicaltrials.gov/study/NCT05151484 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Unknown status | 01/09/2005 | https://clinicaltrials.gov/study/NCT00668941 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/01/2013 | https://clinicaltrials.gov/study/NCT01753856 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00542425 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00414973 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 2 | Completed | 01/06/2007 | https://clinicaltrials.gov/study/NCT00489918 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Enrolling by invitation | 01/02/2024 | https://clinicaltrials.gov/study/NCT06264609 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/09/2003 | https://clinicaltrials.gov/study/NCT00191802 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Terminated | 01/07/2006 | https://clinicaltrials.gov/study/NCT00446589 | 1 | GoF | protect | due to financial problems |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00191321 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01166958 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=aae667c5-381f-4f92-93df-2ed6158d07b0 | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2003 | https://clinicaltrials.gov/study/NCT01611571 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoarthritis, knee | TERIPARATIDE | targetBased | 2 | Completed | 01/02/2010 | https://clinicaltrials.gov/study/NCT01063504 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoarthritis, knee | TERIPARATIDE | targetBased | 2 | Completed | 01/05/2017 | https://clinicaltrials.gov/study/NCT03072147 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 3 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01473589 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 3 | Recruiting | 23/12/2019 | https://clinicaltrials.gov/study/NCT04196855 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 3 | Completed | 24/05/2021 | https://clinicaltrials.gov/study/NCT04921124 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 3 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT01473602 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 4 | Unknown status | 01/10/2016 | https://clinicaltrials.gov/study/NCT02955056 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 4 | Enrolling by invitation | 08/12/2022 | https://clinicaltrials.gov/study/NCT04589819 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 3 | Completed | 01/07/2013 | https://clinicaltrials.gov/study/NCT01896011 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 4 | Not yet recruiting | 01/10/2020 | https://clinicaltrials.gov/study/NCT04533984 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | TERIPARATIDE | targetBased | 4 | Unknown status | 01/07/2010 | https://clinicaltrials.gov/study/NCT01173081 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | humerus fracture | TERIPARATIDE | targetBased | 4 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01105832 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | Osteopenia | TERIPARATIDE | targetBased | 3 | Completed | 01/12/2003 | https://clinicaltrials.gov/study/NCT00065637 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | spinal stenosis | TERIPARATIDE | targetBased | 4 | Completed | 01/08/2012 | https://clinicaltrials.gov/study/NCT02090244 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | neurogenic arthropathy | TERIPARATIDE | targetBased | 2 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02023411 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteogenesis imperfecta | TERIPARATIDE | targetBased | 2 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT01679080 | 0.2 | GoF | protect | Eli Lilly has withdrawn support to the study of teriparatide and placebo pens. The study was not able to continue as a randomized study without the supply of placebo pens. |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteogenesis imperfecta | TERIPARATIDE | targetBased | 4 | Completed | 01/06/2005 | https://clinicaltrials.gov/study/NCT00131469 | 1 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE SULFATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01551069 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | systemic lupus erythematosus | HYDROXYCHLOROQUINE SULFATE | targetBased | 2 | Terminated | 20/11/2013 | https://clinicaltrials.gov/study/NCT01946880 | 0.2 | LoF | protect | Slow enrollment. |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | cutaneous lupus erythematosus | HYDROXYCHLOROQUINE SULFATE | targetBased | 3 | Completed | 01/03/2012 | https://clinicaltrials.gov/study/NCT01551069 | 0.7 | LoF | protect | |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | systemic lupus erythematosus | MEMANTINE HYDROCHLORIDE | targetBased | 2 | Recruiting | 23/08/2018 | https://clinicaltrials.gov/study/NCT03527472 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | sickle cell anemia | MEMANTINE HYDROCHLORIDE | targetBased | 2 | Recruiting | 02/02/2018 | https://clinicaltrials.gov/study/NCT03247218 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | idiopathic scoliosis | KETAMINE HYDROCHLORIDE | targetBased | 2 | Completed | 01/01/2012 | https://clinicaltrials.gov/study/NCT02571491 | 0.2 | LoF | protect | ||
| uHTS identification of small molecule modulators of NR3A | GRIN3A | GRIN3A | bone fracture | KETAMINE HYDROCHLORIDE | targetBased | 3 | Completed | 05/07/2018 | https://clinicaltrials.gov/study/NCT03525821 | 0.7 | LoF | protect | ||
| Inhibitors of the EP2 Prostaglandin E2 Receptor - Primary Screen | PTGER2 | PTGER2 | Prostaglandin E2 receptor EP2 subtype | tibia fracture | EVATANEPAG | targetBased | 2 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00533377 | 0.2 | GoF | protect | |
| Modulators of the EP2 prostaglandin E2 receptor - Primary Screening | PTGER2 | PTGER2 | Prostaglandin E2 receptor EP2 subtype | tibia fracture | EVATANEPAG | targetBased | 2 | Completed | 01/01/2008 | https://clinicaltrials.gov/study/NCT00533377 | 0.2 | GoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINORELBINE | targetBased | 3 | Active, not recruiting | 01/06/2016 | https://clinicaltrials.gov/study/NCT02567435 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINORELBINE | targetBased | 3 | Recruiting | 14/09/2021 | https://clinicaltrials.gov/study/NCT04994132 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | childhood pleomorphic rhabdomyosarcoma | VINORELBINE | targetBased | 2 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01222715 | 0.2 | LoF | protect | |
| Thrombin 1536 HTS | F2_modulation | F2 | Prothrombin | total joint arthroplasty | DABIGATRAN ETEXILATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | spindle cell rhabdomyosarcoma | VINORELBINE TARTRATE | targetBased | 3 | Recruiting | 14/09/2021 | https://clinicaltrials.gov/study/NCT04994132 | 0.7 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | childhood pleomorphic rhabdomyosarcoma | VINORELBINE TARTRATE | targetBased | 2 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01222715 | 0.2 | LoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | DP001 | targetBased | 2 | Completed | 01/03/2007 | https://clinicaltrials.gov/study/NCT00715676 | 0.2 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | MOMELOTINIB | targetBased | 3 | Completed | 06/12/2013 | https://clinicaltrials.gov/study/NCT01969838 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | MOMELOTINIB | targetBased | 2 | Completed | 30/04/2014 | https://clinicaltrials.gov/study/NCT02124746 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | MOMELOTINIB | targetBased | 2 | Completed | 01/11/2010 | https://clinicaltrials.gov/study/NCT01236638 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | MOMELOTINIB | targetBased | 3 | Completed | 19/06/2014 | https://clinicaltrials.gov/study/NCT02101268 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Chagas cardiomyopathy | AMIODARONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 12/06/2017 | https://clinicaltrials.gov/study/NCT03193749 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE HYDROCHLORIDE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE HYDROCHLORIDE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | AL amyloidosis | AMIODARONE HYDROCHLORIDE | targetBased | 2 | Terminated | 01/01/2012 | https://clinicaltrials.gov/study/NCT01511263 | 0.2 | LoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | osteoarthritis | AMLODIPINE BESYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=322f926c-99ac-49f8-9cc5-584594f2891f | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ec636fad-9537-4001-a691-c7b4cc2b6a96 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0038f081-a867-43ed-8415-9f0003871291 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Muscle spasm | CYCLOBENZAPRINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=06da53af-5b46-4e32-a64c-9677e27ae229 | 1 | LoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | bone fracture | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | bone fracture | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | TOREMIFENE CITRATE | targetBased | 3 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00129142 | 0.7 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | osteoarthritis | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | osteoarthritis | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | sinusitis | SEVOFLURANE | targetBased | 4 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02578862 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | bone fracture | SEVOFLURANE | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02621255 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | avascular necrosis | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | rotator cuff tear | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | bone fracture | SEVOFLURANE | targetBased | 4 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02621255 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | rotator cuff tear | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | avascular necrosis | SEVOFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | sinusitis | SEVOFLURANE | targetBased | 4 | Completed | 01/07/2015 | https://clinicaltrials.gov/study/NCT02578862 | 1 | protect | ||
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | Muscle weakness | GUANIDINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=903fbd33-e5d9-41fb-9414-7bd6f42a8593 | 1 | LoF | protect | |||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | osteoarthritis | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | osteoarthritis | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | avascular necrosis | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | rotator cuff tear | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | KCNK3 | Potassium channel subfamily K member 3 | rotator cuff tear | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | KCNK9 | two-pore domain potassium channel | avascular necrosis | DESFLURANE | targetBased | 4 | Completed | 01/09/2016 | https://clinicaltrials.gov/study/NCT03540030 | 1 | protect | ||
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | spinal stenosis | OXYMORPHONE HYDROCHLORIDE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | OXYMORPHONE HYDROCHLORIDE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | spinal stenosis | OXYMORPHONE HYDROCHLORIDE | targetBased | 4 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00652093 | 1 | GoF | protect | Removal of Darvocet from US market |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | METHADONE HYDROCHLORIDE | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | idiopathic scoliosis | METHADONE HYDROCHLORIDE | targetBased | 2 | Completed | 01/09/2013 | https://clinicaltrials.gov/study/NCT01795495 | 0.2 | GoF | protect | |
| qHTS Assay for Identifying the Cell-Membrane Permeable IMPase Inhibitors: Potentiation with Lithium | IMPA1 | IMPA1 | Inositol monophosphatase 1 | spinal cord injury | LITHIUM CARBONATE | targetBased | 2 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00750061 | 0.2 | LoF | protect | |
| qHTS Assay for Identifying the Cell-Membrane Permeable IMPase Inhibitors: Potentiation with Lithium | IMPA1 | IMPA1 | Inositol monophosphatase 1 | bone fracture | LITHIUM CARBONATE | targetBased | 2 | Recruiting | 21/07/2017 | https://clinicaltrials.gov/study/NCT02999022 | 0.2 | LoF | protect | |
| Luminescence Cell-Free Homogenous Primary HTS to Identify Inhibitors of GSK-3 alpha | GSK3A | GSK3A | Glycogen synthase kinase-3 alpha | spinal cord injury | LITHIUM CARBONATE | targetBased | 2 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00750061 | 0.2 | LoF | protect | |
| Luminescence Cell-Free Homogenous Primary HTS to Identify Inhibitors of GSK-3 alpha | GSK3A | GSK3A | Glycogen synthase kinase-3 alpha | bone fracture | LITHIUM CARBONATE | targetBased | 2 | Recruiting | 21/07/2017 | https://clinicaltrials.gov/study/NCT02999022 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | OXYCODONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | OXYCODONE HYDROCHLORIDE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00254631 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | OXYCODONE HYDROCHLORIDE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00254631 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE HYDROCHLORIDE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01366014 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODONE HYDROCHLORIDE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01366014 | 0.2 | GoF | protect | |
| qHTS Assay for Inhibitors of Mammalian Selenoprotein Thioredoxin Reductase 1 (TrxR1): qHTS | TXNRD1 | TXNRD1 | Thioredoxin reductase 1, cytoplasmic | systemic lupus erythematosus | ARSENIC TRIOXIDE | targetBased | 2 | Terminated | 01/07/2013 | https://clinicaltrials.gov/study/NCT01738360 | 0.2 | LoF | protect | Difficulty of inclusions and a sufficient number of relevant clinical information |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROPIPATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdbf5b-b572-448f-80e9-b3e812959901 | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROPIPATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdbf5b-b572-448f-80e9-b3e812959901 | 1 | GoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRROLATE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRROLATE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRROLATE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRROLATE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRROLATE | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Sciatica | CODEINE PHOSPHATE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | CODEINE PHOSPHATE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Sciatica | CODEINE PHOSPHATE | targetBased | 3 | Completed | 28/09/2021 | https://clinicaltrials.gov/study/NCT05626140 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Muscle spasm | CODEINE PHOSPHATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Muscle spasm | CODEINE PHOSPHATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Muscle spasm | CODEINE PHOSPHATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03cdc2f8-78d1-11dc-8314-0800200c9a66 | 1 | GoF | protect | |||
| uHTS identification of DNMT1 inhibitors in a Fluorescent Molecular Beacon assay | DNMT1 | DNMT1 | DNA methyltransferase-1 | myelofibrosis | DECITABINE | targetBased | 2 | Terminated | 01/03/2008 | https://clinicaltrials.gov/study/NCT00630994 | 0.2 | LoF | protect | Stopped due to slow accrual |
| uHTS identification of DNMT1 inhibitors in a Fluorescent Molecular Beacon assay | DNMT1 | DNMT1 | DNA methyltransferase-1 | sickle cell anemia | DECITABINE | targetBased | 2 | Recruiting | 07/07/2022 | https://clinicaltrials.gov/study/NCT05405114 | 0.2 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | NALBUPHINE HYDROCHLORIDE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | NALBUPHINE HYDROCHLORIDE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | NALBUPHINE HYDROCHLORIDE | targetBased | 2 | Unknown status | 01/01/2016 | https://clinicaltrials.gov/study/NCT03034382 | 0.2 | GoF | protect | |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | fibromyalgia | ARMODAFINIL | targetBased | 4 | Completed | 01/08/2007 | https://clinicaltrials.gov/study/NCT00678691 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | temporomandibular joint disorder | BENZTROPINE | targetBased | 2 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00066937 | 0.2 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | temporomandibular joint disorder | BENZTROPINE | targetBased | 2 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00066937 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | temporomandibular joint disorder | BENZTROPINE | targetBased | 2 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00066937 | 0.2 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | temporomandibular joint disorder | BENZTROPINE | targetBased | 2 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00066937 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | temporomandibular joint disorder | BENZTROPINE | targetBased | 2 | Completed | 01/11/2002 | https://clinicaltrials.gov/study/NCT00066937 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Sciatica | BENZTROPINE | targetBased | 2 | Completed | 01/04/1999 | https://clinicaltrials.gov/study/NCT00018200 | 0.2 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Sciatica | BENZTROPINE | targetBased | 2 | Completed | 01/04/1999 | https://clinicaltrials.gov/study/NCT00018200 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Sciatica | BENZTROPINE | targetBased | 2 | Completed | 01/04/1999 | https://clinicaltrials.gov/study/NCT00018200 | 0.2 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Sciatica | BENZTROPINE | targetBased | 2 | Completed | 01/04/1999 | https://clinicaltrials.gov/study/NCT00018200 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Sciatica | BENZTROPINE | targetBased | 2 | Completed | 01/04/1999 | https://clinicaltrials.gov/study/NCT00018200 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | lower extremity fracture | GLYCOPYRRONIUM | targetBased | 4 | Unknown status | 15/09/2017 | https://clinicaltrials.gov/study/NCT03580889 | 1 | LoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE ACETATE | targetBased | 2 | Completed | 01/11/1998 | https://clinicaltrials.gov/study/NCT00018447 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | TERIPARATIDE ACETATE | targetBased | 2 | Completed | 01/05/2004 | https://clinicaltrials.gov/study/NCT00086619 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | TERIPARATIDE ACETATE | targetBased | 2 | Completed | 01/05/2004 | https://clinicaltrials.gov/study/NCT00086619 | 0.2 | GoF | protect | |
| Natriuretic polypeptide receptor (hNpr1) antagonism - Primary qHTS | NPR1 | NPR1 | Atrial natriuretic peptide receptor 1 | cardiomyopathy | NESIRITIDE | targetBased | 3 | Completed | 01/01/2001 | https://clinicaltrials.gov/study/NCT00270387 | 0.7 | GoF | protect | |
| HTS for developing T Cell Immune Modulators | ITGAL | ITGAL | Integrin alpha L, Integrin alpha-L , VWFA domain-containing protein | lupus erythematosus | EFALIZUMAB | targetBased | 2 | Withdrawn | 01/03/2006 | https://clinicaltrials.gov/study/NCT00308204 | 0.2 | LoF | protect | inadequate number of enrolled study subjects |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | interstitial cystitis | ESTROGENS, CONJUGATED | targetBased | 3 | Recruiting | 01/12/2022 | https://clinicaltrials.gov/study/NCT05658874 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | interstitial cystitis | ESTROGENS, CONJUGATED | targetBased | 3 | Recruiting | 01/12/2022 | https://clinicaltrials.gov/study/NCT05658874 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | Osteopenia | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | GoF | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 4 | Completed | 01/11/1997 | https://clinicaltrials.gov/study/NCT00392093 | 1 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/04/1996 | https://clinicaltrials.gov/study/NCT00000419 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | GoF | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | GoF | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | GoF | protect | |||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | interstitial cystitis | ESTROGENS, CONJUGATED | targetBased | 3 | Recruiting | 01/12/2022 | https://clinicaltrials.gov/study/NCT05658874 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | bone disease | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | bone disease | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | bone disease | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 4 | Completed | 01/11/1997 | https://clinicaltrials.gov/study/NCT00392093 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 4 | Completed | 01/11/1997 | https://clinicaltrials.gov/study/NCT00392093 | 1 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/04/1996 | https://clinicaltrials.gov/study/NCT00000419 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | systemic lupus erythematosus | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/04/1996 | https://clinicaltrials.gov/study/NCT00000419 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00000611 | 0.7 | GoF | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/08/1993 | https://clinicaltrials.gov/study/NCT00004650 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=751b6213-92d8-40f8-a2d5-83e8dad086dd | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | Osteopenia | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00000466 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00000611 | 0.7 | GoF | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00000611 | 0.7 | GoF | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=751b6213-92d8-40f8-a2d5-83e8dad086dd | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=751b6213-92d8-40f8-a2d5-83e8dad086dd | 1 | GoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/08/1993 | https://clinicaltrials.gov/study/NCT00004650 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/08/1993 | https://clinicaltrials.gov/study/NCT00004650 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00242710 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | ESTROGENS, CONJUGATED | targetBased | 3 | Terminated | 01/10/1999 | https://clinicaltrials.gov/study/NCT00000430 | 0.7 | GoF | protect | |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | ABL1 | Tyrosine-protein kinase ABL1 | systemic scleroderma | NILOTINIB HYDROCHLORIDE MONOHYDRATE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01166139 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | joint disease | TAPENTADOL | targetBased | 3 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02604446 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | joint disease | TAPENTADOL | targetBased | 3 | Completed | 01/09/2015 | https://clinicaltrials.gov/study/NCT02604446 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | TAPENTADOL | targetBased | 4 | Completed | 05/07/2021 | https://clinicaltrials.gov/study/NCT05999890 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | TAPENTADOL | targetBased | 4 | Completed | 05/07/2021 | https://clinicaltrials.gov/study/NCT05999890 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL | targetBased | 3 | Completed | 01/11/2006 | https://clinicaltrials.gov/study/NCT00361504 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TAPENTADOL | targetBased | 3 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00784277 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TAPENTADOL | targetBased | 3 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00784277 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Completed | 05/07/2021 | https://clinicaltrials.gov/study/NCT05999890 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Completed | 05/07/2021 | https://clinicaltrials.gov/study/NCT05999890 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TAPENTADOL HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/08/2012 | https://clinicaltrials.gov/study/NCT01631513 | 1 | GoF | protect | It was a business decision to cancel this study in Aug. 2012. |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | sterile multifocal osteomyelitis with periostitis and pustulosis | RILONACEPT | pathwayBased | 2 | Completed | 12/02/2013 | https://clinicaltrials.gov/study/NCT01801449 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | familial Mediterranean fever | RILONACEPT | pathwayBased | 2 | Completed | 01/08/2008 | https://clinicaltrials.gov/study/NCT00582907 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00855920 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00610363 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 3 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00958438 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 3 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00856206 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 3 | Completed | 01/02/2009 | https://clinicaltrials.gov/study/NCT00829829 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | RILONACEPT | pathwayBased | 3 | Terminated | 01/11/2011 | https://clinicaltrials.gov/study/NCT01459796 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | cryopyrin-associated periodic syndrome | RILONACEPT | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=282f4099-e848-432a-bac1-e041c192a5ba | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | cryopyrin-associated periodic syndrome | RILONACEPT | pathwayBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/rilonacept-regeneron-previously-arcalyst | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | juvenile idiopathic arthritis | RILONACEPT | pathwayBased | 2 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00534495 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Muckle-Wells syndrome | RILONACEPT | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=282f4099-e848-432a-bac1-e041c192a5ba | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Muckle-Wells syndrome | RILONACEPT | pathwayBased | 3 | Completed | 01/12/2005 | https://clinicaltrials.gov/study/NCT00288704 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | psoriatic arthritis | CANAKINUMAB | pathwayBased | 4 | Recruiting | 15/11/2021 | https://clinicaltrials.gov/study/NCT05080218 | 1 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Schnitzler syndrome | CANAKINUMAB | pathwayBased | 2 | Completed | 01/01/2011 | https://clinicaltrials.gov/study/NCT01276522 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Schnitzler syndrome | CANAKINUMAB | pathwayBased | 2 | Completed | 01/07/2011 | https://clinicaltrials.gov/study/NCT01390350 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Schnitzler syndrome | CANAKINUMAB | pathwayBased | 2 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01245127 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | adult-onset Still's disease | CANAKINUMAB | pathwayBased | 3 | Active, not recruiting | 30/03/2021 | https://clinicaltrials.gov/study/NCT04717635 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | adult-onset Still's disease | CANAKINUMAB | pathwayBased | 2 | Terminated | 21/06/2012 | https://clinicaltrials.gov/study/NCT02204293 | 0.2 | LoF | protect | Recruitment issues due to marketing authorization of study drug |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | mevalonate kinase deficiency | CANAKINUMAB | pathwayBased | 2 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01303380 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | mevalonate kinase deficiency | CANAKINUMAB | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d271f3b-e4f9-4d80-8dcf-28d49123f80e | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | familial Mediterranean fever | CANAKINUMAB | pathwayBased | 2 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01088880 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | familial Mediterranean fever | CANAKINUMAB | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d271f3b-e4f9-4d80-8dcf-28d49123f80e | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | familial Mediterranean fever | CANAKINUMAB | pathwayBased | 2 | Completed | 01/12/2010 | https://clinicaltrials.gov/study/NCT01148797 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis | CANAKINUMAB | pathwayBased | 2 | Completed | 01/04/2010 | https://clinicaltrials.gov/study/NCT01160822 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | sickle cell anemia | CANAKINUMAB | pathwayBased | 2 | Completed | 05/04/2017 | https://clinicaltrials.gov/study/NCT02961218 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | CINCA syndrome | CANAKINUMAB | pathwayBased | 3 | Terminated | 26/01/2009 | https://clinicaltrials.gov/study/NCT00770601 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis, knee | CANAKINUMAB | pathwayBased | 2 | Active, not recruiting | 27/08/2021 | https://clinicaltrials.gov/study/NCT04814368 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00889863 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Completed | 07/05/2015 | https://clinicaltrials.gov/study/NCT02396212 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Terminated | 01/07/2009 | https://clinicaltrials.gov/study/NCT00886769 | 0.35 | LoF | protect | recommendation by Data Monitoring Committee |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Withdrawn | 01/01/2013 | https://clinicaltrials.gov/study/NCT01676948 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Completed | 01/09/2009 | https://clinicaltrials.gov/study/NCT00891046 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Completed | 03/11/2014 | https://clinicaltrials.gov/study/NCT02334748 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | systemic juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 3 | Completed | 17/11/2014 | https://clinicaltrials.gov/study/NCT02296424 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Withdrawn | 01/09/2012 | https://clinicaltrials.gov/study/NCT01593527 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Completed | 01/11/2011 | https://clinicaltrials.gov/study/NCT01470989 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Completed | 01/12/2009 | https://clinicaltrials.gov/study/NCT01029652 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01356602 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 2 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00663169 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Completed | 25/08/2011 | https://clinicaltrials.gov/study/NCT01431638 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 2 | Completed | 05/06/2009 | https://clinicaltrials.gov/study/NCT00927810 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 2 | Completed | 01/12/2008 | https://clinicaltrials.gov/study/NCT00819585 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 2 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00798369 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Terminated | 20/06/2011 | https://clinicaltrials.gov/study/NCT01362608 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | gout | CANAKINUMAB | pathwayBased | 3 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01080131 | 0.7 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | cryopyrin-associated periodic syndrome | CANAKINUMAB | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d271f3b-e4f9-4d80-8dcf-28d49123f80e | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | cryopyrin-associated periodic syndrome | CANAKINUMAB | pathwayBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/ilaris | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | juvenile idiopathic arthritis | CANAKINUMAB | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d271f3b-e4f9-4d80-8dcf-28d49123f80e | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | Muckle-Wells syndrome | CANAKINUMAB | pathwayBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7d271f3b-e4f9-4d80-8dcf-28d49123f80e | 1 | LoF | protect | |||
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | pulmonary sarcoidosis | CANAKINUMAB | pathwayBased | 2 | Completed | 19/12/2016 | https://clinicaltrials.gov/study/NCT02888080 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | TRAMADOL | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01598753 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | TRAMADOL | targetBased | 3 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01598753 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | TRAMADOL | targetBased | 4 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT02850211 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | TRAMADOL | targetBased | 4 | Completed | 01/09/2011 | https://clinicaltrials.gov/study/NCT02850211 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | TRAMADOL | targetBased | 2 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT03380533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | TRAMADOL | targetBased | 2 | Completed | 22/09/2016 | https://clinicaltrials.gov/study/NCT03380533 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | TRAMADOL | targetBased | 4 | Completed | 21/04/2021 | https://clinicaltrials.gov/study/NCT04837924 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | TRAMADOL | targetBased | 4 | Completed | 21/04/2021 | https://clinicaltrials.gov/study/NCT04837924 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | TRAMADOL | targetBased | 2 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01630343 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | TRAMADOL | targetBased | 2 | Completed | 01/01/2010 | https://clinicaltrials.gov/study/NCT01630343 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TRAMADOL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00833911 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TRAMADOL | targetBased | 3 | Completed | 01/04/2003 | https://clinicaltrials.gov/study/NCT00833911 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TRAMADOL | targetBased | 2 | Completed | 30/08/2018 | https://clinicaltrials.gov/study/NCT03850587 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | TRAMADOL | targetBased | 2 | Completed | 30/08/2018 | https://clinicaltrials.gov/study/NCT03850587 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | TRAMADOL | targetBased | 4 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02110966 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | TRAMADOL | targetBased | 4 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02110966 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | TRAMADOL | targetBased | 4 | Completed | 21/06/2021 | https://clinicaltrials.gov/study/NCT05488925 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | pulpitis | TRAMADOL | targetBased | 4 | Completed | 21/06/2021 | https://clinicaltrials.gov/study/NCT05488925 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ankylosing spondylitis | TRAMADOL | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00647517 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ankylosing spondylitis | TRAMADOL | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT00647517 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01019265 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/03/2008 | https://clinicaltrials.gov/study/NCT01019265 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Recruiting | 01/01/2019 | https://clinicaltrials.gov/study/NCT03781544 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Recruiting | 01/01/2019 | https://clinicaltrials.gov/study/NCT03781544 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT01728246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT01728246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT01063842 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT01063842 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00635349 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00635349 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL | targetBased | 2 | Completed | 21/07/2016 | https://clinicaltrials.gov/study/NCT02845271 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | TRAMADOL | targetBased | 3 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01920386 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | TRAMADOL | targetBased | 3 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01920386 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Inguinal hernia | TRAMADOL | targetBased | 4 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT01943760 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Inguinal hernia | TRAMADOL | targetBased | 4 | Completed | 01/09/2012 | https://clinicaltrials.gov/study/NCT01943760 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | HYDROMORPHONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | HYDROMORPHONE HYDROCHLORIDE | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00399048 | 0.7 | GoF | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE HYDROCHLORIDE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | HYDROMORPHONE HYDROCHLORIDE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00635349 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Completed | 01/05/2007 | https://clinicaltrials.gov/study/NCT00635349 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT01063842 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | TRAMADOL HYDROCHLORIDE | targetBased | 4 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT01063842 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | TRAMADOL HYDROCHLORIDE | targetBased | 3 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01920386 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | toothache | TRAMADOL HYDROCHLORIDE | targetBased | 3 | Completed | 01/06/2013 | https://clinicaltrials.gov/study/NCT01920386 | 0.7 | GoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | scoliosis | CLEVIDIPINE | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01645111 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | FEDRATINIB | targetBased | 3 | Completed | 27/03/2019 | https://clinicaltrials.gov/study/NCT03755518 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | FEDRATINIB | targetBased | 2 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT01692366 | 0.2 | LoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | muscular disease | ALFACALCIDOL | targetBased | 3 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT02327091 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ALFACALCIDOL | targetBased | 4 | Completed | 01/08/2011 | https://clinicaltrials.gov/study/NCT01430104 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ALFACALCIDOL | targetBased | 2 | Recruiting | 12/10/2017 | https://clinicaltrials.gov/study/NCT03183557 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ALFACALCIDOL | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00144456 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ALFACALCIDOL | targetBased | 4 | Unknown status | 01/01/2013 | https://clinicaltrials.gov/study/NCT01765010 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ALFACALCIDOL | targetBased | 3 | Completed | 01/05/2000 | https://clinicaltrials.gov/study/NCT00138983 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | scoliosis | ALFACALCIDOL | targetBased | 2 | Recruiting | 20/09/2018 | https://clinicaltrials.gov/study/NCT03582917 | 0.2 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | systemic lupus erythematosus | ALFACALCIDOL | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00668330 | 1 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | postmenopausal osteoporosis | ALFACALCIDOL | targetBased | 3 | Completed | 01/05/2005 | https://clinicaltrials.gov/study/NCT00165698 | 0.7 | GoF | protect | |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | sarcopenia | ALFACALCIDOL | targetBased | 4 | Not yet recruiting | 13/02/2024 | https://clinicaltrials.gov/study/NCT06272227 | 1 | GoF | protect | |
| Thrombin 1536 HTS | F2_modulation | F2 | Prothrombin | total joint arthroplasty | DABIGATRAN ETEXILATE MESYLATE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/pradaxa | 1 | LoF | protect | |||
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | systemic lupus erythematosus | BRENTUXIMAB VEDOTIN | targetBased | 2 | Terminated | 01/07/2015 | https://clinicaltrials.gov/study/NCT02533570 | 0.2 | LoF | protect | Sponsor decision based on portfolio prioritization |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | diffuse scleroderma | BRENTUXIMAB VEDOTIN | targetBased | 2 | Recruiting | 14/02/2024 | https://clinicaltrials.gov/study/NCT05149768 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | diffuse scleroderma | BRENTUXIMAB VEDOTIN | targetBased | 2 | Completed | 07/05/2019 | https://clinicaltrials.gov/study/NCT03198689 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis | GEVOKIZUMAB | pathwayBased | 2 | Completed | 01/07/2012 | https://clinicaltrials.gov/study/NCT01683396 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis | GEVOKIZUMAB | pathwayBased | 2 | Completed | 01/05/2013 | https://clinicaltrials.gov/study/NCT01882491 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | Hypereosinophilic syndrome | RUXOLITINIB | targetBased | 2 | Recruiting | 26/09/2002 | https://clinicaltrials.gov/study/NCT00044304 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | discoid lupus erythematosus | RUXOLITINIB | targetBased | 2 | Completed | 04/05/2022 | https://clinicaltrials.gov/study/NCT04908280 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | discoid lupus erythematosus | RUXOLITINIB | targetBased | 2 | Recruiting | 19/04/2024 | https://clinicaltrials.gov/study/NCT06261021 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 20/08/2020 | https://clinicaltrials.gov/study/NCT04218071 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 3 | Completed | 06/12/2013 | https://clinicaltrials.gov/study/NCT01969838 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 29/09/2016 | https://clinicaltrials.gov/study/NCT02784496 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Active, not recruiting | 28/08/2018 | https://clinicaltrials.gov/study/NCT03427866 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Terminated | 03/05/2021 | https://clinicaltrials.gov/study/NCT04543279 | 0.2 | LoF | protect | Low accrual |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Recruiting | 16/12/2021 | https://clinicaltrials.gov/study/NCT05037760 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Terminated | 01/11/2013 | https://clinicaltrials.gov/study/NCT01790295 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 4 | Completed | 01/05/2012 | https://clinicaltrials.gov/study/NCT01558739 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 3 | Completed | 16/08/2011 | https://clinicaltrials.gov/study/NCT01493414 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 01/10/2013 | https://clinicaltrials.gov/study/NCT01981850 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 3 | Recruiting | 11/03/2021 | https://clinicaltrials.gov/study/NCT04562389 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Active, not recruiting | 20/10/2014 | https://clinicaltrials.gov/study/NCT02251821 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 3 | Terminated | 03/02/2016 | https://clinicaltrials.gov/study/NCT02598297 | 0.7 | LoF | protect | Unresolvable inability to recruit the patients. |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 3 | Completed | 01/07/2009 | https://clinicaltrials.gov/study/NCT00934544 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 30/06/2011 | https://clinicaltrials.gov/study/NCT01369498 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 4 | Recruiting | 10/06/2022 | https://clinicaltrials.gov/study/NCT05447260 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 21/02/2013 | https://clinicaltrials.gov/study/NCT01712308 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Active, not recruiting | 27/02/2017 | https://clinicaltrials.gov/study/NCT03069326 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | RUXOLITINIB | targetBased | 2 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01340651 | 0.2 | LoF | protect | |
| Primary biochemical high-throughput screening assay for inhibitors of Rho kinase 2 (Rhok2) | ROCK2 | ROCK2 | Rho-associated protein kinase 2 | diffuse scleroderma | BELUMOSUDIL | targetBased | 2 | Terminated | 03/03/2021 | https://clinicaltrials.gov/study/NCT04680975 | 0.2 | LoF | protect | Sponsor decision due to slow enrollment and strategic consideration; not driven by any safety concerns. |
| Primary biochemical high-throughput screening assay for inhibitors of Rho kinase 2 (Rhok2) | ROCK2 | ROCK2 | Rho-associated protein kinase 2 | diffuse scleroderma | BELUMOSUDIL | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT03919799 | 0.2 | LoF | protect | Sponsor decision due to slow enrollment and strategic consideration; not driven by any safety concerns. |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myelofibrosis | PACRITINIB | targetBased | 2 | Active, not recruiting | 04/06/2018 | https://clinicaltrials.gov/study/NCT03645824 | 0.2 | LoF | protect | |
| Fluorescence-based biochemical primary high throughput screening assay to identify activators of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | TNNI3 | Troponin I, cardiac muscle | muscular disease | LEVOSIMENDAN | targetBased | 2 | Unknown status | 01/09/2012 | https://clinicaltrials.gov/study/NCT01721434 | 0.2 | GoF | protect | |
| Fluorescence-based biochemical primary high throughput screening assay to identify inhibitors of the calcium sensitivity of cardiac Regulated Thin Filaments (RTF) | TNNI3 | TNNI3 | Troponin I, cardiac muscle | muscular disease | LEVOSIMENDAN | targetBased | 2 | Unknown status | 01/09/2012 | https://clinicaltrials.gov/study/NCT01721434 | 0.2 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | TOFACITINIB CITRATE | targetBased | 2 | Recruiting | 26/04/2023 | https://clinicaltrials.gov/study/NCT05814939 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | TOFACITINIB CITRATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=68e3d6b2-7838-4d2d-a417-09d919b43e13 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | MORPHINE SULFATE | targetBased | 4 | Unknown status | 01/09/2009 | https://clinicaltrials.gov/study/NCT01281891 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, hip | MORPHINE SULFATE | targetBased | 4 | Unknown status | 01/09/2009 | https://clinicaltrials.gov/study/NCT01281891 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE SULFATE | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT03693404 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bone fracture | MORPHINE SULFATE | targetBased | 4 | Completed | 01/12/2018 | https://clinicaltrials.gov/study/NCT03693404 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE SULFATE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01904071 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | hip fracture | MORPHINE SULFATE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT01904071 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 2 | Unknown status | 01/08/2015 | https://clinicaltrials.gov/study/NCT02967302 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 2 | Unknown status | 01/08/2015 | https://clinicaltrials.gov/study/NCT02967302 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00901628 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 4 | Completed | 01/04/2008 | https://clinicaltrials.gov/study/NCT00901628 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 2 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02860949 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | MORPHINE SULFATE | targetBased | 2 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02860949 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 4 | Completed | 15/03/2015 | https://clinicaltrials.gov/study/NCT02222246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/03/2011 | https://clinicaltrials.gov/study/NCT00880373 | 1 | GoF | protect | The funding withdrawal and early termination of the trial is based upon lack of suitable recruitment figures in order to reach the required trial endpoints. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 2 | Completed | 01/11/2019 | https://clinicaltrials.gov/study/NCT04301336 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | sickle cell anemia | MORPHINE SULFATE | targetBased | 2 | Completed | 01/11/2019 | https://clinicaltrials.gov/study/NCT04301336 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/03/2014 | https://clinicaltrials.gov/study/NCT02063997 | 0.2 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gout | ARHALOFENATE | targetBased | 2 | Completed | 01/06/2011 | https://clinicaltrials.gov/study/NCT01399008 | 0.2 | protect | ||
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | fibromyalgia | DROXIDOPA | targetBased | 2 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01323374 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | FAXELADOL | targetBased | 2 | Completed | 19/09/2005 | https://clinicaltrials.gov/study/NCT03783910 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fibromyalgia | FAXELADOL | targetBased | 2 | Completed | 19/09/2005 | https://clinicaltrials.gov/study/NCT03783910 | 0.2 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile dermatomyositis | BARICITINIB | targetBased | 2 | Recruiting | 10/11/2022 | https://clinicaltrials.gov/study/NCT05524311 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | myositis | BARICITINIB | targetBased | 2 | Completed | 07/10/2021 | https://clinicaltrials.gov/study/NCT04208464 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | BARICITINIB | targetBased | 3 | Completed | 02/08/2018 | https://clinicaltrials.gov/study/NCT03616964 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | BARICITINIB | targetBased | 2 | Completed | 24/03/2016 | https://clinicaltrials.gov/study/NCT02708095 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | BARICITINIB | targetBased | 3 | Terminated | 09/09/2019 | https://clinicaltrials.gov/study/NCT03843125 | 0.35 | LoF | protect | Study terminated due to insufficient evidence to support a positive benefit: risk profile. Safety findings were consistent with previously published OLUMIANT data |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | BARICITINIB | targetBased | 3 | Terminated | 02/08/2018 | https://clinicaltrials.gov/study/NCT03616912 | 0.35 | LoF | protect | Study terminated due to insufficient evidence to support a positive benefit: risk profile in systemic lupus erythematosus patients. |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic scleroderma | BARICITINIB | targetBased | 4 | Recruiting | 08/03/2022 | https://clinicaltrials.gov/study/NCT05300932 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | dermatomyositis | BARICITINIB | targetBased | 2 | Withdrawn | 01/06/2022 | https://clinicaltrials.gov/study/NCT05361109 | 0.2 | LoF | protect | Difficulty recruiting subjects |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | dermatomyositis | BARICITINIB | targetBased | 3 | Recruiting | 31/08/2022 | https://clinicaltrials.gov/study/NCT04972760 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | BARICITINIB | targetBased | 4 | Recruiting | 15/11/2021 | https://clinicaltrials.gov/study/NCT05080218 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | Aicardi-Goutieres syndrome | BARICITINIB | targetBased | 2 | Completed | 03/06/2019 | https://clinicaltrials.gov/study/NCT03921554 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic juvenile idiopathic arthritis | BARICITINIB | targetBased | 3 | Recruiting | 12/02/2020 | https://clinicaltrials.gov/study/NCT04088396 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | BARICITINIB | targetBased | 3 | Completed | 17/12/2018 | https://clinicaltrials.gov/study/NCT03773978 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | BARICITINIB | targetBased | 3 | Recruiting | 05/04/2019 | https://clinicaltrials.gov/study/NCT03773965 | 0.7 | LoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bfdd5d5b-7569-4848-afa2-e820fbe3c8fb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e16705d8-4472-4f83-96ac-69fa2be066cb | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e1b75458-2e5b-46b9-92c6-fa6daba3770f | 1 | protect | ||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/07/2001 | https://clinicaltrials.gov/study/NCT00481169 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | osteoporosis | BAZEDOXIFENE ACETATE | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00205777 | 0.7 | protect | ||
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | KEAP1 | Kelch-like ECH-associated protein 1 | psoriatic arthritis | DIMETHYL FUMARATE | targetBased | 2 | Withdrawn | 01/05/2015 | https://clinicaltrials.gov/study/NCT02475304 | 0.2 | LoF | protect | Difficulties to enrol patients |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of myeloid cell leukemia sequence 1 (MCL1) interactions with BIM-BH3 peptide. | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX MESYLATE | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| uHTS of Mcl-1/Bid interaction inhibitors | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX MESYLATE | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| uHTS of Mcl-1/Noxa interaction inhibitors | MCL1 | MCL1 | Induced myeloid leukemia cell differentiation protein Mcl-1 , MCL1 apoptosis regulator, BCL2 family member, Myeloid cell leukemia ES variant | myelofibrosis | OBATOCLAX MESYLATE | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Multiplexed high-throughput screen for small molecule regulators of Bcl-2 family protein interactions, specifically Bim-Bfl-1 | BCL2A1_modulators | BCL2A1 | Bcl-2-related protein A1 | myelofibrosis | OBATOCLAX MESYLATE | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Primary biochemical high throughput screening assay to identify inhibitors of BCL2-related protein, long isoform (BCLXL). | BCL2L1_modulators | BCL2L1 | Bcl-2-like protein 1 | myelofibrosis | OBATOCLAX MESYLATE | targetBased | 2 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00360035 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal cord injury | NERISPIRDINE | targetBased | 2 | Completed | 01/10/2004 | https://clinicaltrials.gov/study/NCT00093275 | 0.2 | LoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | PARATHYROID HORMONE | targetBased | 4 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00583518 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | PARATHYROID HORMONE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/natpar | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 10/01/2005 | https://clinicaltrials.gov/study/NCT00172120 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 01/09/1999 | https://clinicaltrials.gov/study/NCT00000427 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/10/1999 | https://clinicaltrials.gov/study/NCT00005005 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/09/1987 | https://clinicaltrials.gov/study/NCT00005006 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 4 | Recruiting | 01/07/2019 | https://clinicaltrials.gov/study/NCT03994172 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 4 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00479037 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 27/04/2000 | https://clinicaltrials.gov/study/NCT00172081 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 4 | Completed | 01/09/2005 | https://clinicaltrials.gov/study/NCT00221299 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/05/2008 | https://clinicaltrials.gov/study/NCT00683163 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/08/1999 | https://clinicaltrials.gov/study/NCT00004993 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 16/10/2001 | https://clinicaltrials.gov/study/NCT00172133 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 18/05/1995 | https://clinicaltrials.gov/study/NCT00172107 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 10/01/2004 | https://clinicaltrials.gov/study/NCT00172172 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 3 | Completed | 01/07/2006 | https://clinicaltrials.gov/study/NCT00365456 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/05/2009 | https://clinicaltrials.gov/study/NCT00853723 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/06/1999 | https://clinicaltrials.gov/study/NCT00021827 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | PARATHYROID HORMONE | targetBased | 2 | Completed | 01/08/1999 | https://clinicaltrials.gov/study/NCT00000400 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | PARATHYROID HORMONE | targetBased | 4 | Unknown status | 01/03/2012 | https://clinicaltrials.gov/study/NCT01687374 | 1 | GoF | protect | |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn6) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | FGB | Fibrinogen beta chain [Cleaved into: Fibrinopeptide B; Fibrinogen beta chain] | osteoarthritis | FIBRINOGEN, HUMAN | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01235715 | 1 | protect | ||
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn7) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | FGB | Fibrinogen beta chain [Cleaved into: Fibrinopeptide B; Fibrinogen beta chain] | osteoarthritis | FIBRINOGEN, HUMAN | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01235715 | 1 | protect | ||
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | dermatomyositis | SIPONIMOD | targetBased | 2 | Terminated | 15/06/2010 | https://clinicaltrials.gov/study/NCT01148810 | 0.2 | GoF | protect | Trial was prematurely terminated due to enrollment challenges. |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | polymyositis | SIPONIMOD | targetBased | 2 | Terminated | 24/04/2013 | https://clinicaltrials.gov/study/NCT01801917 | 0.2 | GoF | protect | |
| qHTS Assay for Inhibitors of Mammalian Selenoprotein Thioredoxin Reductase 1 (TrxR1): qHTS | TXNRD1 | TXNRD1 | Thioredoxin reductase 1, cytoplasmic | rheumatic disease | AUROTHIOGLUCOSE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=M01CB04 | 1 | LoF | protect | |||
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | rotator cuff tear | CLOMIPHENE | targetBased | 2 | Recruiting | 09/03/2023 | https://clinicaltrials.gov/study/NCT04944836 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | rotator cuff tear | CLOMIPHENE | targetBased | 2 | Recruiting | 09/03/2023 | https://clinicaltrials.gov/study/NCT04944836 | 0.2 | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01357837 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01357837 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 04/12/2012 | https://clinicaltrials.gov/study/NCT01709214 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 04/12/2012 | https://clinicaltrials.gov/study/NCT01709214 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 04/12/2012 | https://clinicaltrials.gov/study/NCT01709214 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis, knee | CEBRANOPADOL | targetBased | 2 | Completed | 01/05/2011 | https://clinicaltrials.gov/study/NCT01357837 | 0.2 | GoF | protect | |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | rotator cuff tear | CLOMIPHENE CITRATE | targetBased | 2 | Recruiting | 09/03/2023 | https://clinicaltrials.gov/study/NCT04944836 | 0.2 | protect | ||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | rotator cuff tear | CLOMIPHENE CITRATE | targetBased | 2 | Recruiting | 09/03/2023 | https://clinicaltrials.gov/study/NCT04944836 | 0.2 | protect | ||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | ABALOPARATIDE | targetBased | 4 | Recruiting | 01/02/2021 | https://clinicaltrials.gov/study/NCT04467983 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | ABALOPARATIDE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/eladynos | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | ABALOPARATIDE | targetBased | 3 | Completed | 05/08/2019 | https://clinicaltrials.gov/study/NCT04064411 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | postmenopausal osteoporosis | ABALOPARATIDE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01343004 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 3 | Completed | 03/05/2018 | https://clinicaltrials.gov/study/NCT03512262 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 2 | Completed | 01/04/2007 | https://clinicaltrials.gov/study/NCT00542425 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208743lbl.pdf | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 3 | Completed | 01/04/2011 | https://clinicaltrials.gov/study/NCT01343004 | 0.7 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=712143d9-e21e-4013-bb3b-3426a21060a8 | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 4 | Active, not recruiting | 10/01/2020 | https://clinicaltrials.gov/study/NCT04167163 | 1 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 2 | Completed | 25/09/2012 | https://clinicaltrials.gov/study/NCT01674621 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | osteoporosis | ABALOPARATIDE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/eladynos | 1 | GoF | protect | |||
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | ABALOPARATIDE | targetBased | 4 | Withdrawn | 01/06/2024 | https://clinicaltrials.gov/study/NCT04626141 | 1 | GoF | protect | Study withdrawn due to staffing/enrollment related challenges. |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | bone fracture | ABALOPARATIDE | targetBased | 2 | Recruiting | 18/05/2022 | https://clinicaltrials.gov/study/NCT04760782 | 0.2 | GoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | fracture of pelvis | ABALOPARATIDE | targetBased | 2 | Completed | 17/09/2020 | https://clinicaltrials.gov/study/NCT04249232 | 0.2 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | FILGOTINIB | targetBased | 2 | Completed | 06/10/2017 | https://clinicaltrials.gov/study/NCT03285711 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | FILGOTINIB | targetBased | 2 | Completed | 07/03/2017 | https://clinicaltrials.gov/study/NCT03117270 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | FILGOTINIB | targetBased | 3 | Withdrawn | 01/12/2020 | https://clinicaltrials.gov/study/NCT04483687 | 0.7 | LoF | protect | Development program terminated |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | FILGOTINIB | targetBased | 3 | Withdrawn | 01/12/2020 | https://clinicaltrials.gov/study/NCT04483700 | 0.7 | LoF | protect | Development program terminated |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | FILGOTINIB | targetBased | 2 | Completed | 09/03/2017 | https://clinicaltrials.gov/study/NCT03101670 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | FILGOTINIB | targetBased | 3 | Terminated | 03/12/2019 | https://clinicaltrials.gov/study/NCT04115748 | 0.7 | LoF | protect | Development program terminated |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | FILGOTINIB | targetBased | 3 | Terminated | 13/11/2019 | https://clinicaltrials.gov/study/NCT04115839 | 0.7 | LoF | protect | Development program terminated |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | FILGOTINIB | targetBased | 2 | Terminated | 26/07/2017 | https://clinicaltrials.gov/study/NCT03320876 | 0.2 | LoF | protect | development program for filgotinib for participants with psoriatic arthritis has been stopped |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | cutaneous lupus erythematosus | FILGOTINIB | targetBased | 2 | Completed | 24/05/2017 | https://clinicaltrials.gov/study/NCT03134222 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | spondyloarthropathy | FILGOTINIB | targetBased | 3 | Active, not recruiting | 05/04/2023 | https://clinicaltrials.gov/study/NCT05785611 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | KCNQ1 | Potassium voltage-gated channel subfamily KQT member 1 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy, type III | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Terminated | 07/03/2019 | https://clinicaltrials.gov/study/NCT03819660 | 0.2 | LoF | protect | Development of indication not being pursued |
| Primary cell-based high-throughput screening assay for identification of compounds that protect hERG from block by proarrhythmic agents | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Wildtype qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of KCHN2 3.1: Mutant qHTS | KCNH2 | KCNH2 | Potassium voltage-gated channel subfamily H member 2 , Voltage-gated inwardly rectifying potassium channel KCNH2 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate KCNQ2 potassium channels | KCNQ2 | KCNQ2 | Potassium voltage-gated channel subfamily KQT member 2 | spinal muscular atrophy | AMIFAMPRIDINE PHOSPHATE | targetBased | 2 | Completed | 21/01/2019 | https://clinicaltrials.gov/study/NCT03781479 | 0.2 | LoF | protect | |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | cutaneous lupus erythematosus | KRP203 | targetBased | 2 | Completed | 01/02/2011 | https://clinicaltrials.gov/study/NCT01294774 | 0.2 | GoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | UPADACITINIB | targetBased | 2 | Completed | 27/07/2020 | https://clinicaltrials.gov/study/NCT04451772 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | systemic lupus erythematosus | UPADACITINIB | targetBased | 2 | Completed | 25/07/2019 | https://clinicaltrials.gov/study/NCT03978520 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | UPADACITINIB | targetBased | 3 | Active, not recruiting | 26/11/2019 | https://clinicaltrials.gov/study/NCT04169373 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | ankylosing spondylitis | UPADACITINIB | targetBased | 2 | Completed | 24/10/2017 | https://clinicaltrials.gov/study/NCT03178487 | 0.2 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | UPADACITINIB | targetBased | 3 | Active, not recruiting | 27/04/2017 | https://clinicaltrials.gov/study/NCT03104400 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | UPADACITINIB | targetBased | 3 | Active, not recruiting | 01/05/2017 | https://clinicaltrials.gov/study/NCT03104374 | 0.7 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | psoriatic arthritis | UPADACITINIB | targetBased | 4 | Recruiting | 15/11/2021 | https://clinicaltrials.gov/study/NCT05080218 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | juvenile idiopathic arthritis | UPADACITINIB | targetBased | 3 | Recruiting | 02/10/2023 | https://clinicaltrials.gov/study/NCT05609630 | 0.7 | LoF | protect | |
| Identification of agents that induce E-selectin on human endothelial cells Measured in Cell-Based System Using Imaging - 2152-01_Activator_SinglePoint_HTS_Activity | SELE | SELE | E-selectin | sickle cell anemia | RIVIPANSEL | targetBased | 3 | Completed | 17/06/2015 | https://clinicaltrials.gov/study/NCT02187003 | 0.7 | LoF | protect | |
| Primary HTS assay for chemical inhibitors of TNF alpha stimulated E-Selectin expression | E-selectinExpression | SELE | E-Selectin | sickle cell anemia | RIVIPANSEL | targetBased | 3 | Completed | 17/06/2015 | https://clinicaltrials.gov/study/NCT02187003 | 0.7 | LoF | protect | |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | NFKB1 | Nuclear factor NF-kappa-B p105 subunit | Duchenne muscular dystrophy | EDASALONEXENT | pathwayBased | 3 | Terminated | 14/03/2019 | https://clinicaltrials.gov/study/NCT03917719 | 0.35 | LoF | protect | The Phase 3 PolarisDMD trial did not meet the primary endpoint. As a result, activities related to the development of edasalonexent have stopped including the CAT-1004-302 Open-Label Study of Edasalonexent in Boys With Duchenne Muscular Dystrophy |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | NFKB1 | Cystic fibrosis transmembrane conductance regulator | Duchenne muscular dystrophy | EDASALONEXENT | pathwayBased | 3 | Terminated | 14/03/2019 | https://clinicaltrials.gov/study/NCT03917719 | 0.35 | LoF | protect | The Phase 3 PolarisDMD trial did not meet the primary endpoint. As a result, activities related to the development of edasalonexent have stopped including the CAT-1004-302 Open-Label Study of Edasalonexent in Boys With Duchenne Muscular Dystrophy |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | NFKB1 | Nuclear factor NF-kappa-B p105 subunit | Duchenne muscular dystrophy | EDASALONEXENT | pathwayBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03703882 | 0.7 | LoF | protect | |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | NFKB1 | Cystic fibrosis transmembrane conductance regulator | Duchenne muscular dystrophy | EDASALONEXENT | pathwayBased | 3 | Completed | 02/10/2018 | https://clinicaltrials.gov/study/NCT03703882 | 0.7 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 4 | Withdrawn | 01/01/2015 | https://clinicaltrials.gov/study/NCT02153177 | 1 | GoF | protect | The study was withdrawn prior to any participants being enrolled. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | rotator cuff tear | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 22/01/2019 | https://clinicaltrials.gov/study/NCT03818919 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis | HYDROCODONE BITARTRATE | targetBased | 2 | Completed | 01/09/2002 | https://clinicaltrials.gov/study/NCT02222740 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | osteoarthritis | DIFELIKEFALIN | targetBased | 2 | Completed | 01/08/2015 | https://clinicaltrials.gov/study/NCT02524197 | 0.2 | GoF | protect | |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | systemic lupus erythematosus | IBERDOMIDE | targetBased | 2 | Completed | 31/08/2017 | https://clinicaltrials.gov/study/NCT03161483 | 0.2 | protect | ||
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | systemic lupus erythematosus | IBERDOMIDE | targetBased | 2 | Completed | 16/09/2014 | https://clinicaltrials.gov/study/NCT02185040 | 0.2 | protect | ||
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | RBX1 | E3 ubiquitin-protein ligase RBX1 | sarcoidosis | IBERDOMIDE | targetBased | 2 | Withdrawn | 01/11/2014 | https://clinicaltrials.gov/study/NCT02192489 | 0.2 | protect | Administrative | |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | NLRP3 | NACHT, LRR and PYD domains-containing protein 3, NACHT, LRR and PYD domains-containing protein 3 | Schnitzler syndrome | DAPANSUTRILE | targetBased | 2 | Terminated | 15/05/2018 | https://clinicaltrials.gov/study/NCT03595371 | 0.2 | LoF | protect | The study was terminated due to study design issues that did not allow for determination of efficacy and safety in subjects with Schnitzler Syndrome who are currently well controlled on anakinra therapy. |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | IFNG | Interferon gamma | adult-onset Still's disease | EMAPALUMAB | targetBased | 3 | Recruiting | 15/12/2021 | https://clinicaltrials.gov/study/NCT05001737 | 0.7 | LoF | protect | |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | IFNG | Interferon gamma | systemic juvenile idiopathic arthritis | EMAPALUMAB | targetBased | 3 | Recruiting | 15/12/2021 | https://clinicaltrials.gov/study/NCT05001737 | 0.7 | LoF | protect | |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | IFNG | Interferon gamma | systemic lupus erythematosus | EMAPALUMAB | targetBased | 3 | Recruiting | 15/12/2021 | https://clinicaltrials.gov/study/NCT05001737 | 0.7 | LoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | osteoarthritis, knee | LIRAGLUTIDE | targetBased | 4 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02910570 | 1 | GoF | protect | |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | osteoarthritis, knee | LIRAGLUTIDE | targetBased | 4 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02910570 | 1 | GoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | osteoarthritis, knee | LIRAGLUTIDE | targetBased | 4 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02910570 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diffuse scleroderma | LANIFIBRANOR | targetBased | 2 | Completed | 29/10/2015 | https://clinicaltrials.gov/study/NCT02503644 | 0.2 | GoF | protect | |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | AKT1 | RAC-alpha serine/threonine-protein kinase | Proteus syndrome | MIRANSERTIB | targetBased | 2 | Recruiting | 20/05/2022 | https://clinicaltrials.gov/study/NCT04316546 | 0.2 | LoF | protect | |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | systemic lupus erythematosus | CENERIMOD | targetBased | 3 | Not yet recruiting | 01/07/2024 | https://clinicaltrials.gov/study/NCT06475742 | 0.7 | protect | ||
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | systemic lupus erythematosus | CENERIMOD | targetBased | 3 | Recruiting | 13/12/2022 | https://clinicaltrials.gov/study/NCT05648500 | 0.7 | protect | ||
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | systemic lupus erythematosus | CENERIMOD | targetBased | 2 | Completed | 21/12/2018 | https://clinicaltrials.gov/study/NCT03742037 | 0.2 | protect | ||
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | systemic lupus erythematosus | CENERIMOD | targetBased | 3 | Recruiting | 26/06/2023 | https://clinicaltrials.gov/study/NCT05672576 | 0.7 | protect | ||
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | discoid lupus erythematosus | DELGOCITINIB | targetBased | 2 | Terminated | 09/07/2019 | https://clinicaltrials.gov/study/NCT03958955 | 0.2 | LoF | protect | Terminated due to recruitment challenges. |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | osteoporosis | ELDECALCITOL | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00144456 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODEGOL | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01619839 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | osteoarthritis, knee | OXYCODEGOL | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01619839 | 0.2 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | TLR9 | Toll-like receptor 9 | dermatomyositis | BAZLITORAN | targetBased | 2 | Completed | 01/11/2015 | https://clinicaltrials.gov/study/NCT02612857 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | AL amyloidosis | BELANTAMAB MAFODOTIN | targetBased | 2 | Recruiting | 26/02/2021 | https://clinicaltrials.gov/study/NCT04617925 | 0.2 | LoF | protect | |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | NFE2L2 | Nuclear factor erythroid 2-related factor 2 | Friedreich ataxia | OMAVELOXOLONE | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216718Orig1s000lbl.pdf | 1 | GoF | protect | |||
| qHTS of Nrf2 Activators | Nrf2 activators | NFE2L2 | NFE2 like bZIP transcription factor 2, Nuclear factor erythroid 2-related factor 2 | Friedreich ataxia | OMAVELOXOLONE | pathwayBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216718Orig1s000lbl.pdf | 1 | GoF | protect | |||
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | NFE2L2 | Nuclear factor erythroid 2-related factor 2 | Friedreich ataxia | OMAVELOXOLONE | targetBased | 2 | Active, not recruiting | 31/01/2015 | https://clinicaltrials.gov/study/NCT02255435 | 0.2 | GoF | protect | |
| qHTS of Nrf2 Activators | Nrf2 activators | NFE2L2 | NFE2 like bZIP transcription factor 2, Nuclear factor erythroid 2-related factor 2 | Friedreich ataxia | OMAVELOXOLONE | pathwayBased | 2 | Active, not recruiting | 31/01/2015 | https://clinicaltrials.gov/study/NCT02255435 | 0.2 | GoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis, hand | LUTIKIZUMAB | pathwayBased | 2 | Completed | 01/03/2015 | https://clinicaltrials.gov/study/NCT02384538 | 0.2 | LoF | protect | |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | IL1B | Interleukin-1 beta | osteoarthritis, knee | LUTIKIZUMAB | pathwayBased | 2 | Completed | 04/06/2014 | https://clinicaltrials.gov/study/NCT02087904 | 0.2 | LoF | protect | |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | PTH1R | Parathyroid hormone/parathyroid hormone-related peptide receptor | hip fracture | OSTABOLIN-C | targetBased | 2 | Withdrawn | 01/12/2008 | https://clinicaltrials.gov/study/NCT00787358 | 0.2 | GoF | protect | Change in company direction |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/fablyn | 1 | protect | |||||
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | ESR1 | Estrogen receptor, Estrogen receptor | postmenopausal osteoporosis | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/fablyn | 1 | protect | |||||
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | ESR2 | Estrogen receptor beta | postmenopausal osteoporosis | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/fablyn | 1 | protect |