A small screen carried out in the ExviTech platform developed by Vivia Biotech resulted in the identification of several oxadiazole derivatives, some of which are recorded in ChEMBL DB.
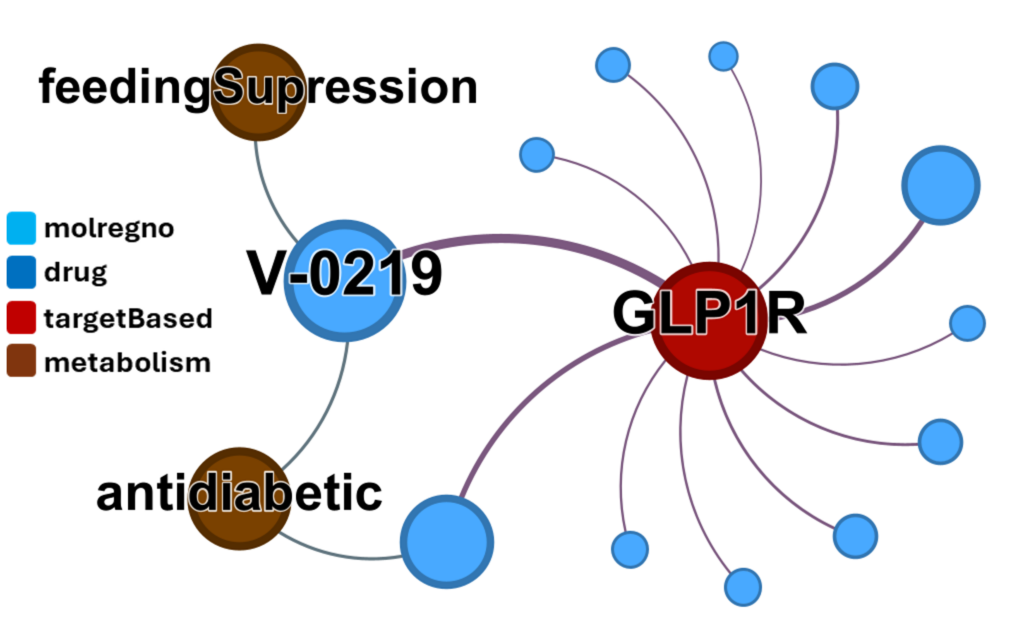
Their ChEMBL interactions depicted in this network graph.
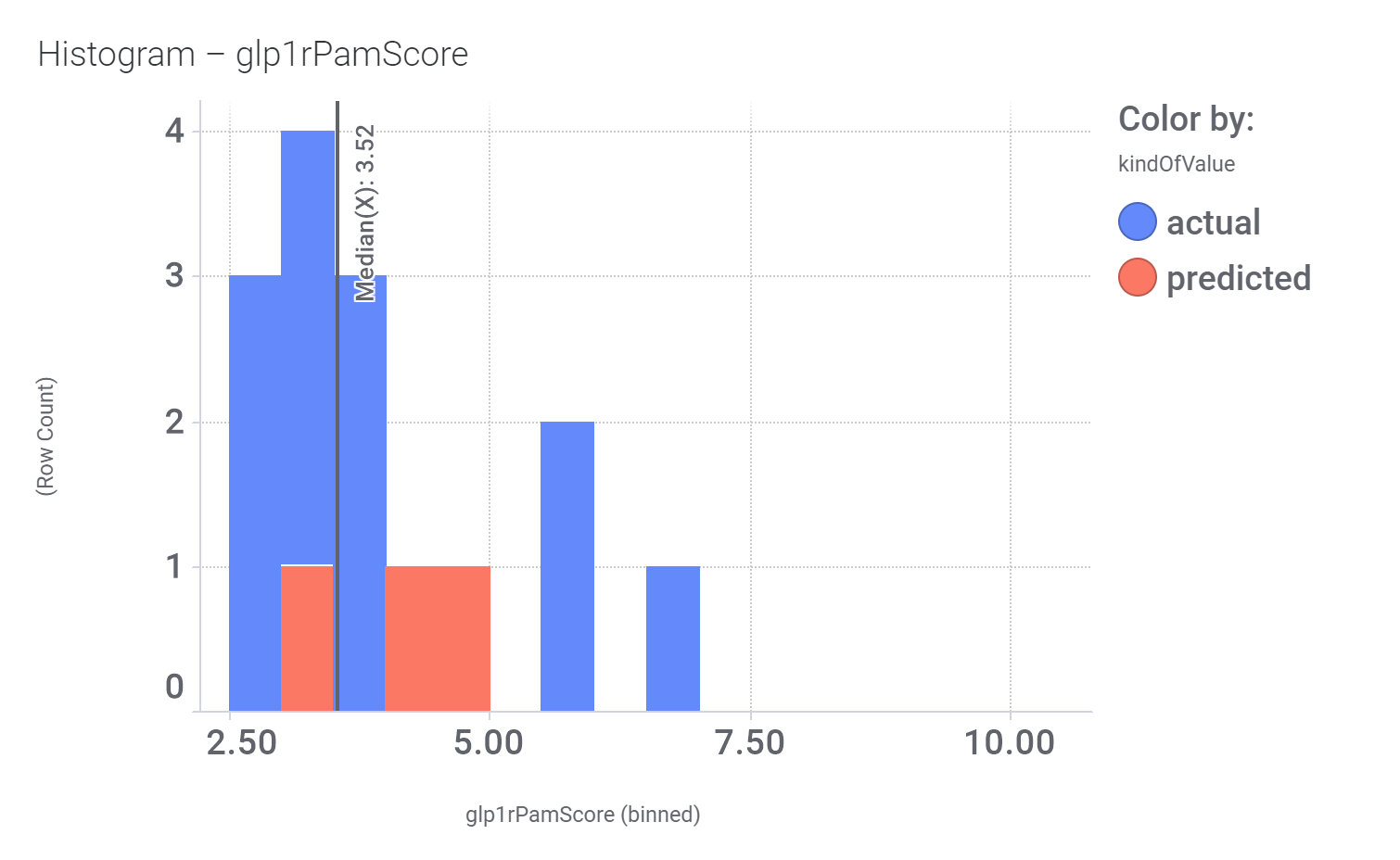
And specific ChEMBL assay where they show activity specified in this treeMap.
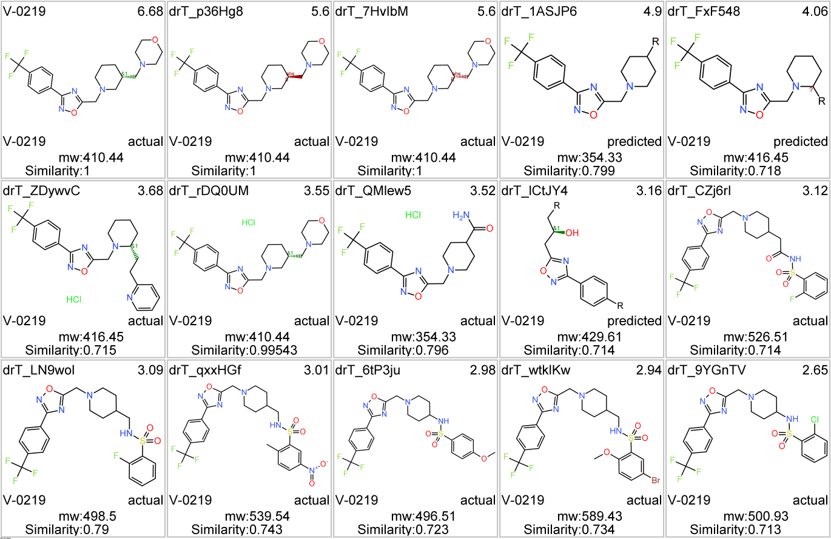
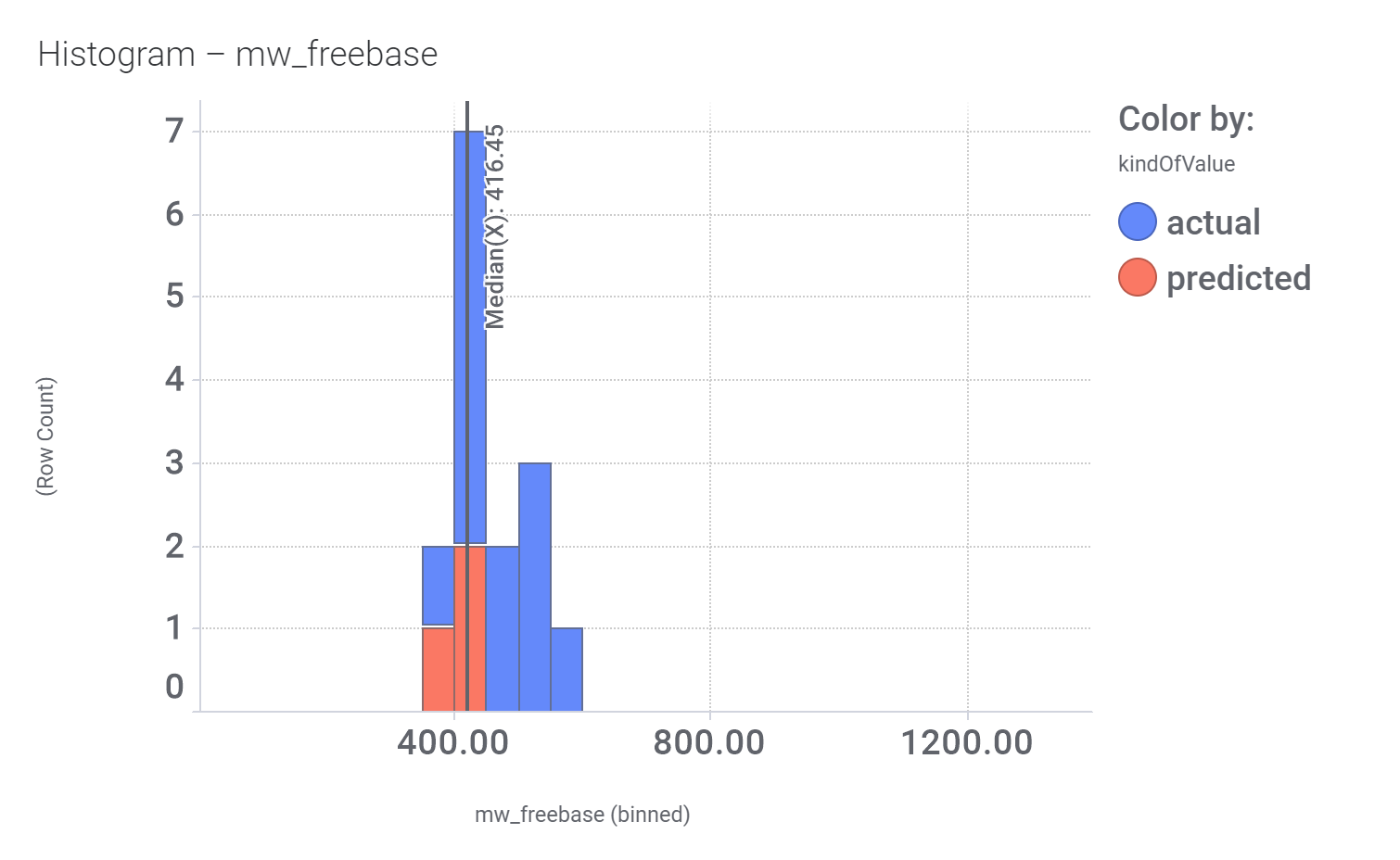
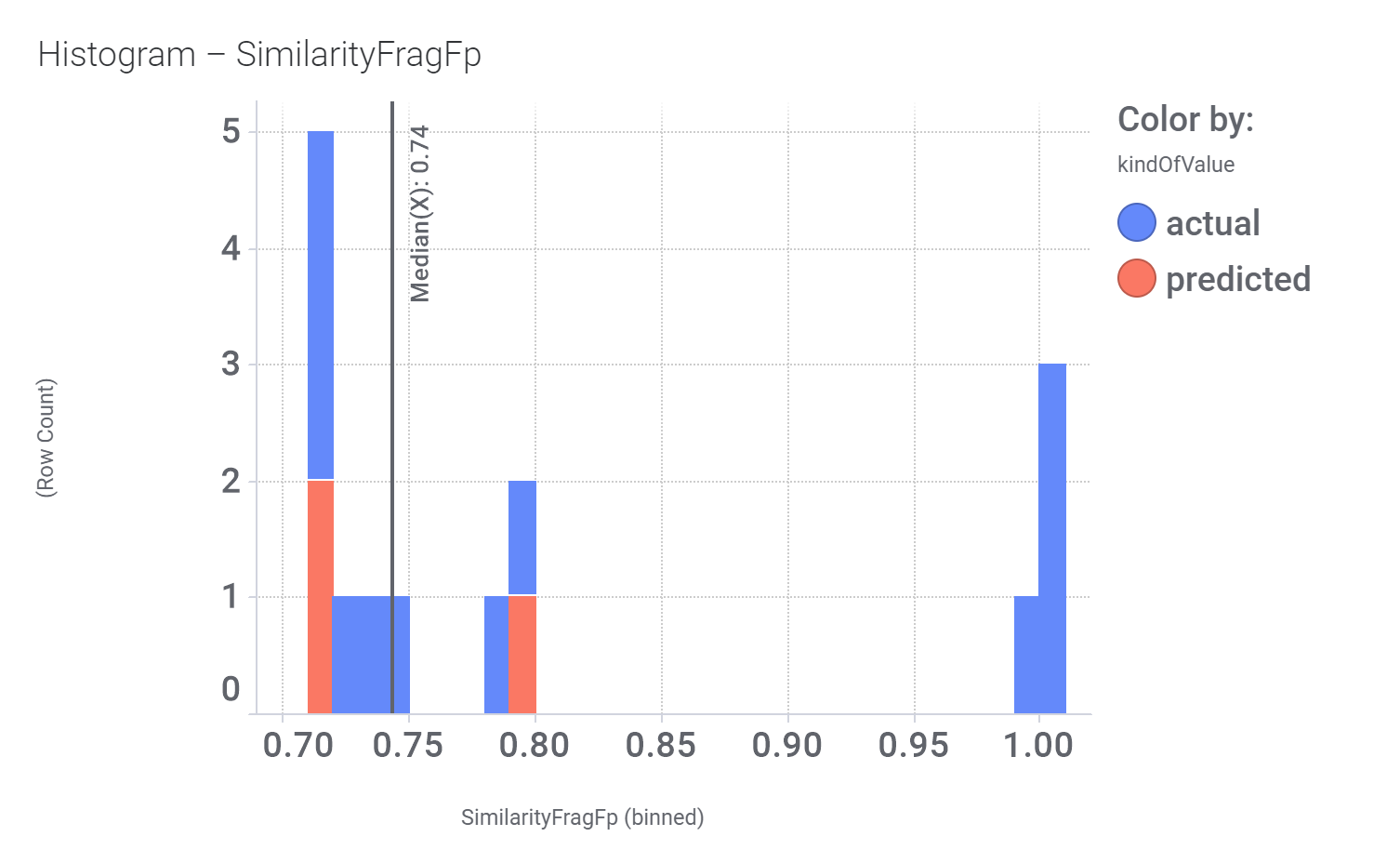
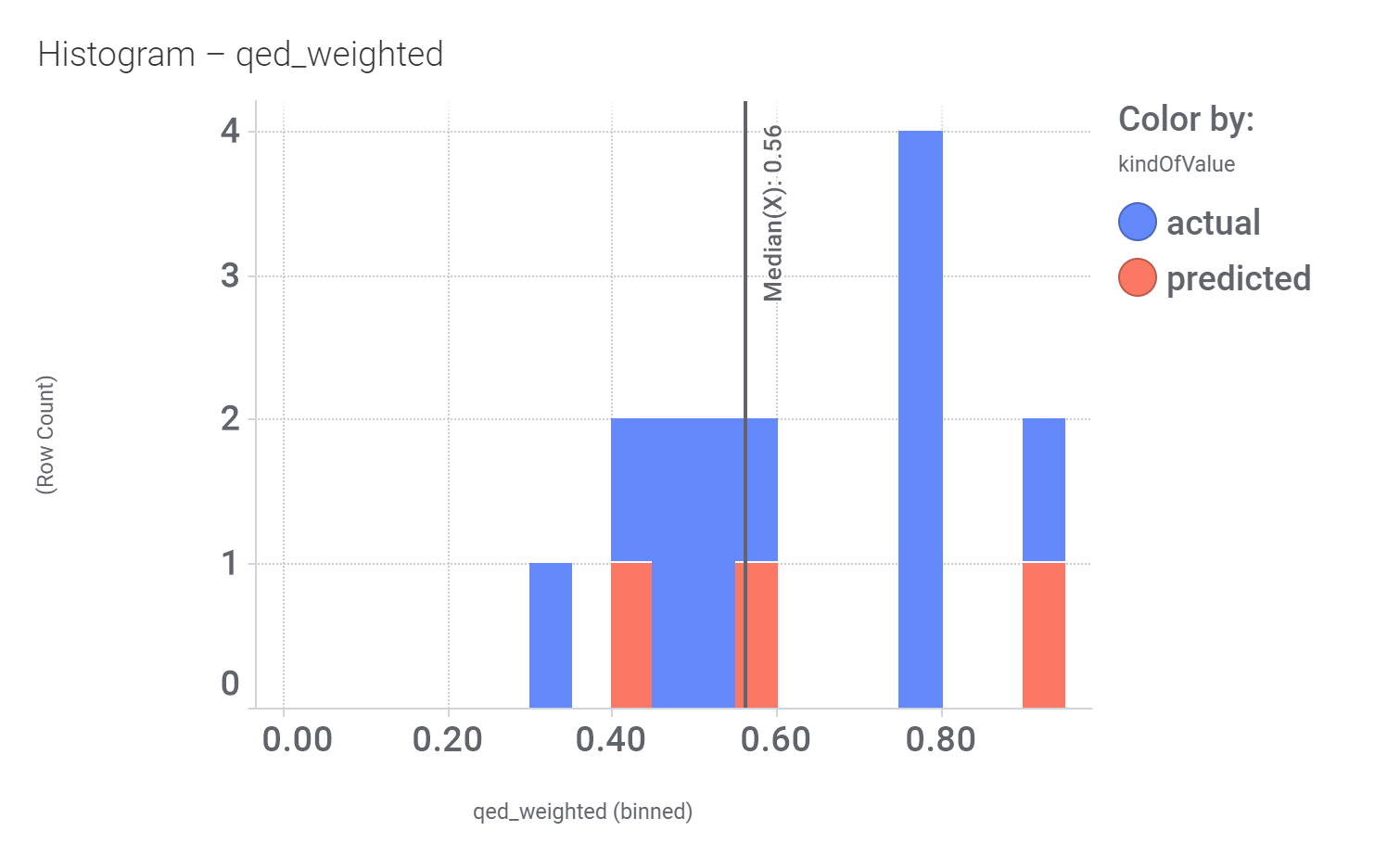
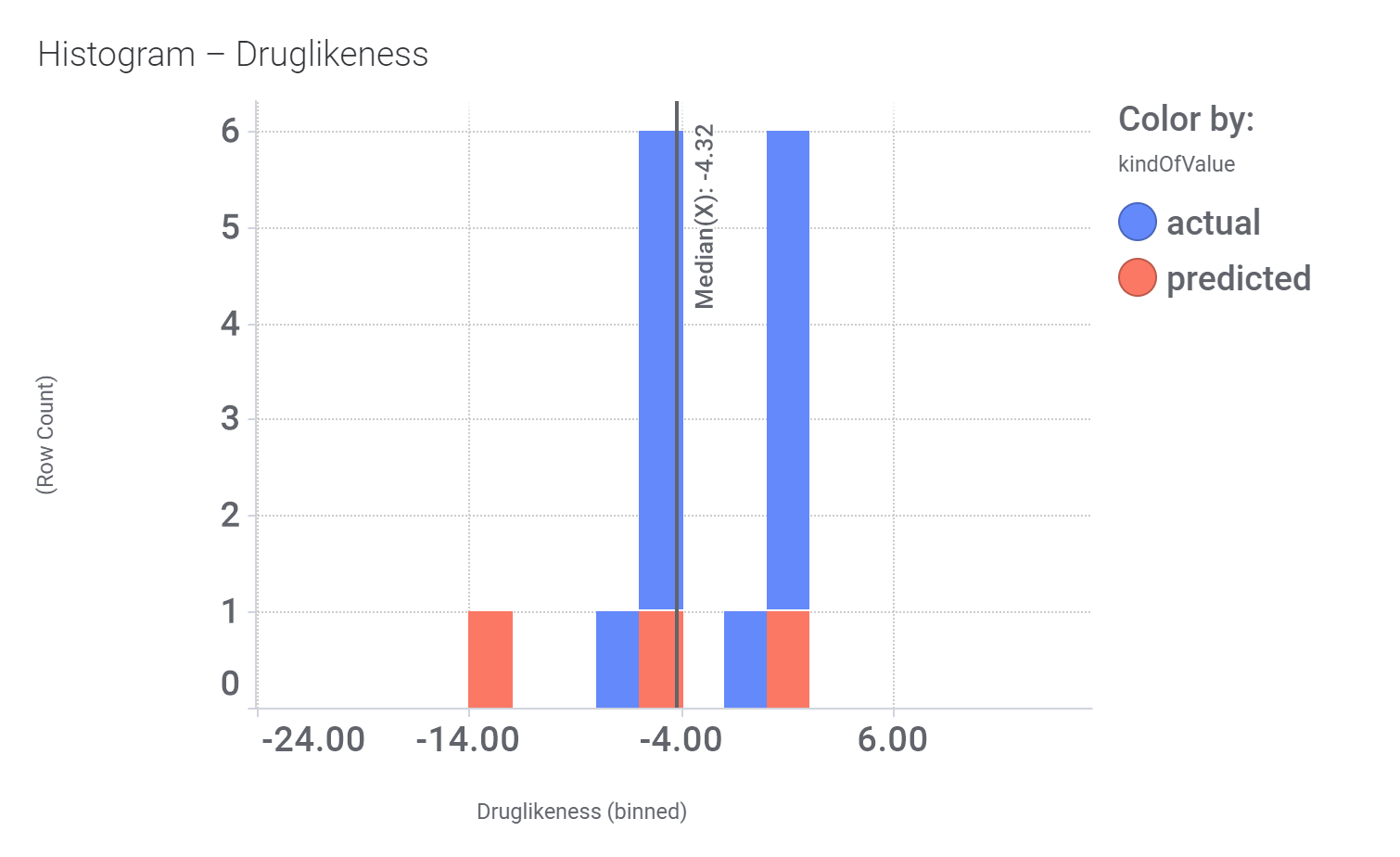
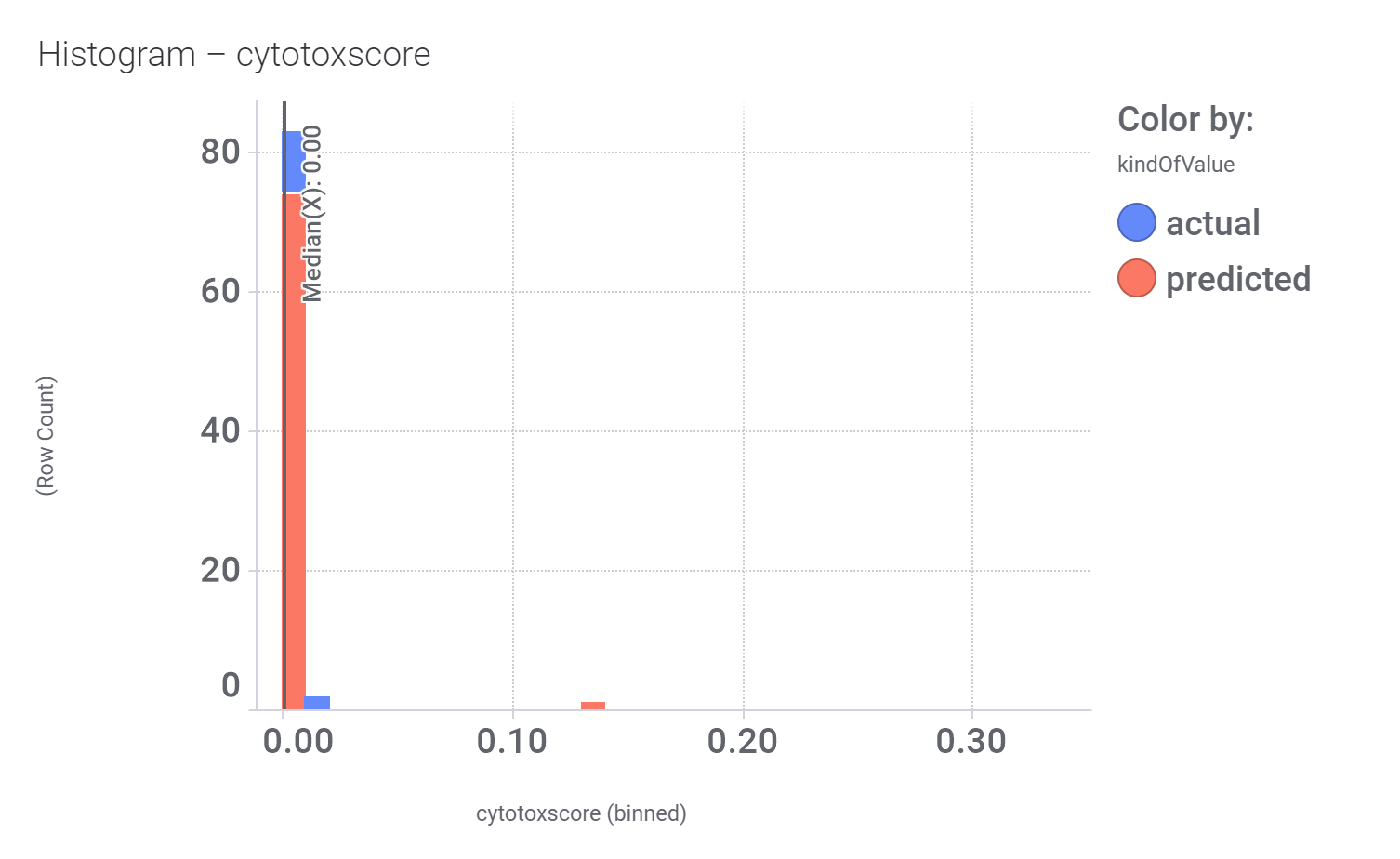
Here, their bio-physicochemical and developability properties.
Druglikeness estimated by QedWeighted
Druglikeness estimated by DataWarrior.
Literature
J Med Chem. 2022 Apr 14; 65(7): 5449–5461.
doi: 10.1021/acs.jmedchem.1c01842, PMCID: PMC9014410. PMID: 35349261
Discovery of V-0219: A Small-Molecule Positive Allosteric Modulator of the Glucagon-Like Peptide-1 Receptor toward Oral Treatment for “Diabesity”
Juan M. Decara,† Henar Vázquez-Villa,‡ José Brea,∥ Mónica Alonso,† Raj Kamal Srivastava,⊥# Laura Orio,∇ Francisco Alén,∇ Juan Suárez,† Elena Baixeras,† Javier García-Cárceles,‡ Andrea Escobar-Peña,‡ Beat Lutz,⊥ Ramón Rodríguez,○ Eva Codesido,○ F. Javier Garcia-Ladona,◆ Teresa A. Bennett,¶§ Juan A. Ballesteros,¶ Jacobo Cruces,○ María I. Loza,∥ Bellinda Benhamú,‡ Fernando Rodríguez de Fonseca,*†∇ and María L. López-Rodríguez*‡
Abstract
Peptidic agonists of the glucagon-like peptide-1 receptor (GLP-1R) have gained a prominent role in the therapy of type-2 diabetes and are being considered for reducing food intake in obesity. Potential advantages of small molecules acting as positive allosteric modulators (PAMs) of GLP-1R, including oral administration and reduced unwanted effects, could improve the utility of this class of drugs. Here, we describe the discovery of compound 9 (4-{[1-({3-[4-(trifluoromethyl)phenyl]-1,2,4-oxadiazol-5-yl}methyl)piperidin-3-yl]methyl}morpholine, V-0219) that exhibits enhanced efficacy of GLP-1R stimulation, subnanomolar potency in the potentiation of insulin secretion, and no significant off-target activities. The identified GLP-1R PAM shows a remarkable in vivo activity, reducing food intake and improving glucose handling in normal and diabetic rodents. Enantioselective synthesis revealed oral efficacy for (S)-9 in animal models. Compound 9 behavior bolsters the interest of a small-molecule PAM of GLP-1R as a promising therapeutic approach for the increasingly prevalent obesity-associated diabetes.