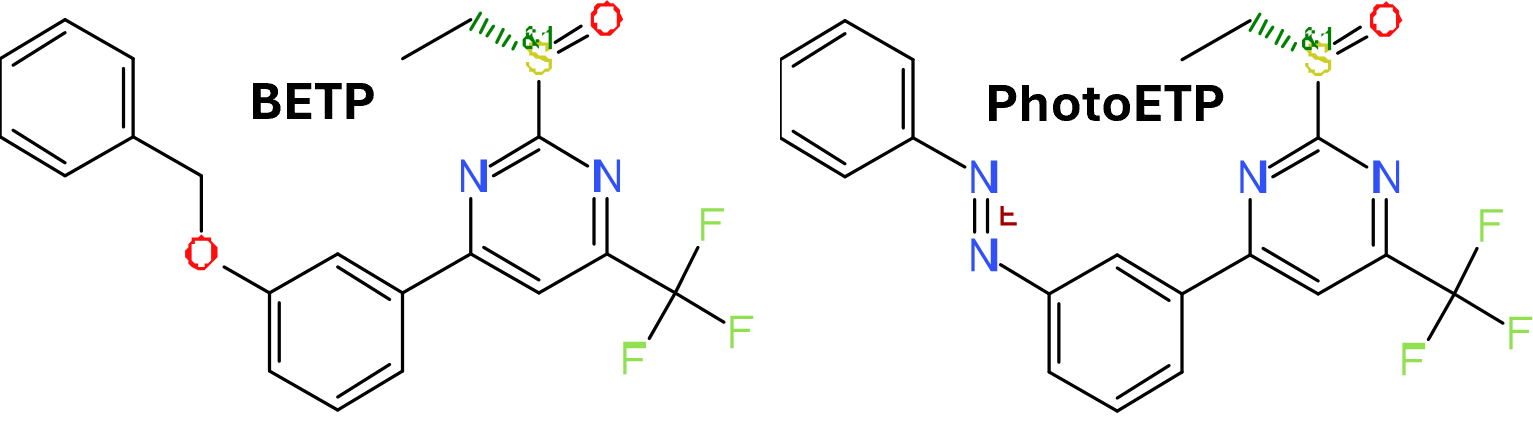
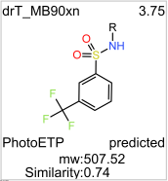
BETP & PhotoETP come from a structure-based library screen carried ut at Lilly. Both share a ethylsulfinylTrifluoromethylPyrimidine core linked to a stilbenoid-type scaffold. DrTarget virtual screen identified just one similar structure with potential GLP1R PAM activity.
In ChEMBL, drT_MB90xn shows weak activity in one assay:
Inhibition of LPS induced NO production in mouse RAW264.7 cells at 30 uM preincubated for 1 hr followed by LPS addition and measured after 24 hrs by Griess reagent based assay relative to control
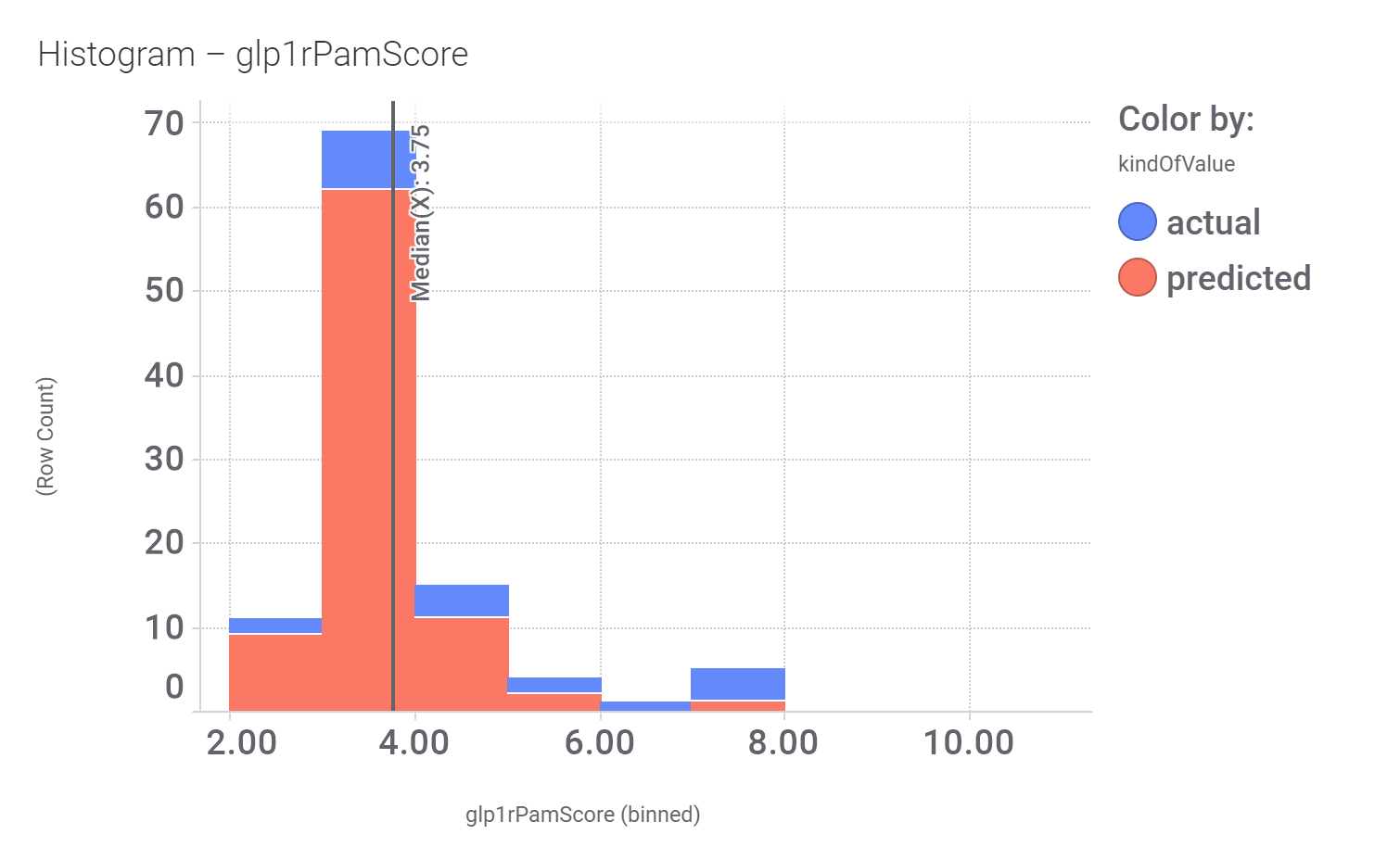
| glp1rPamScore | 3.75 |
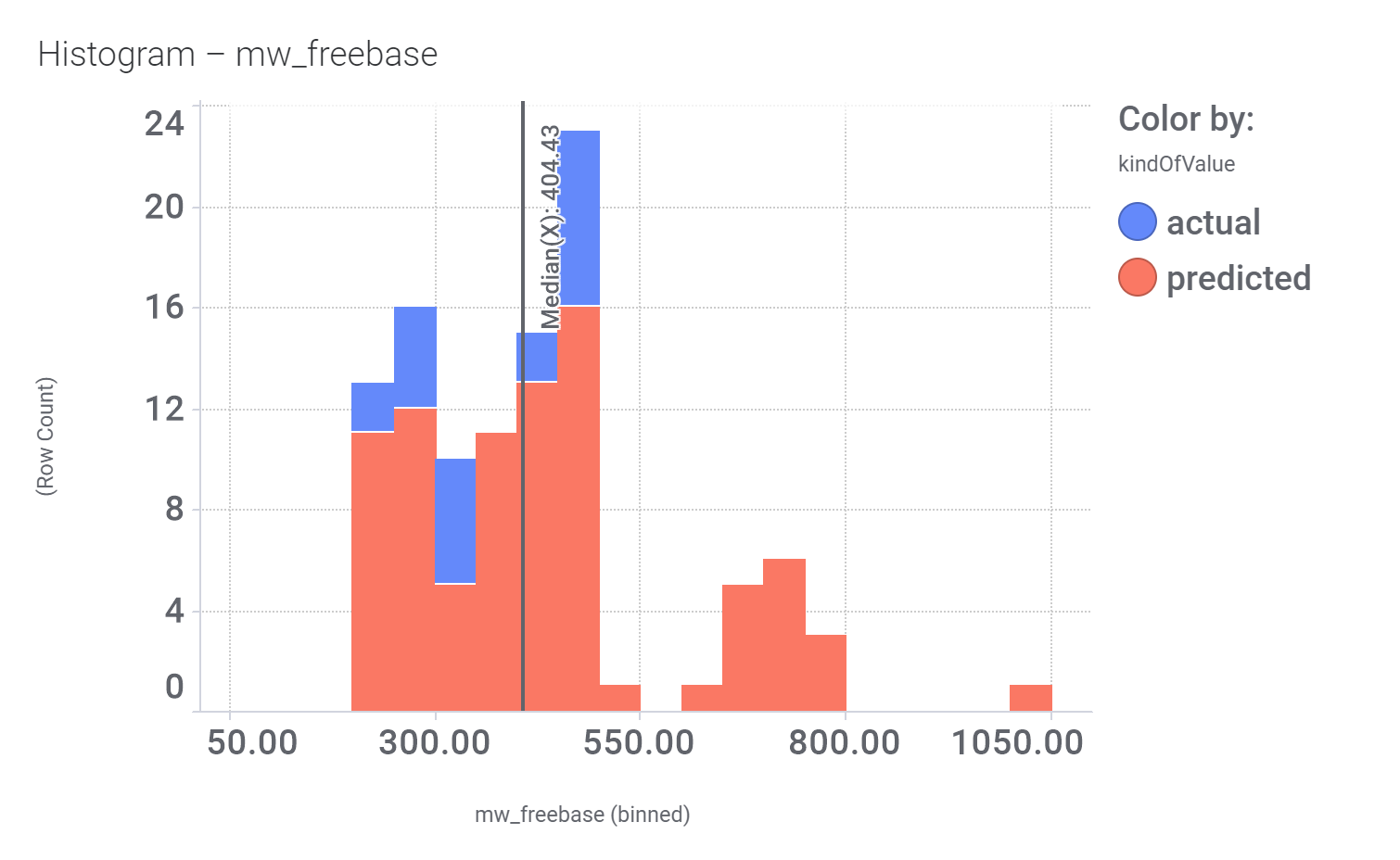
| mw_freebase | 507.52 |
| SimilarityFragFp | 0.74026 |
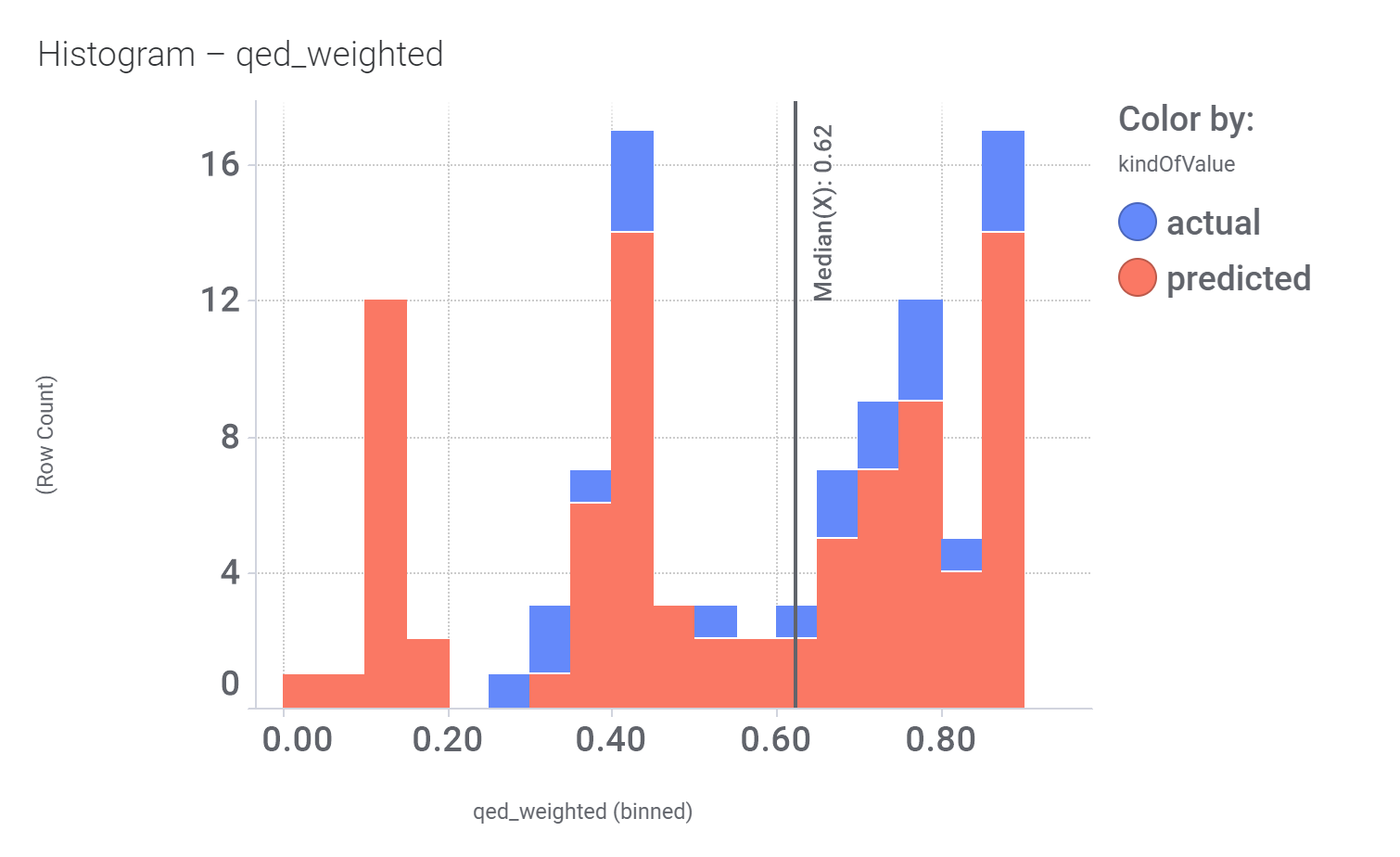
| qed_weighted | 0.39 |
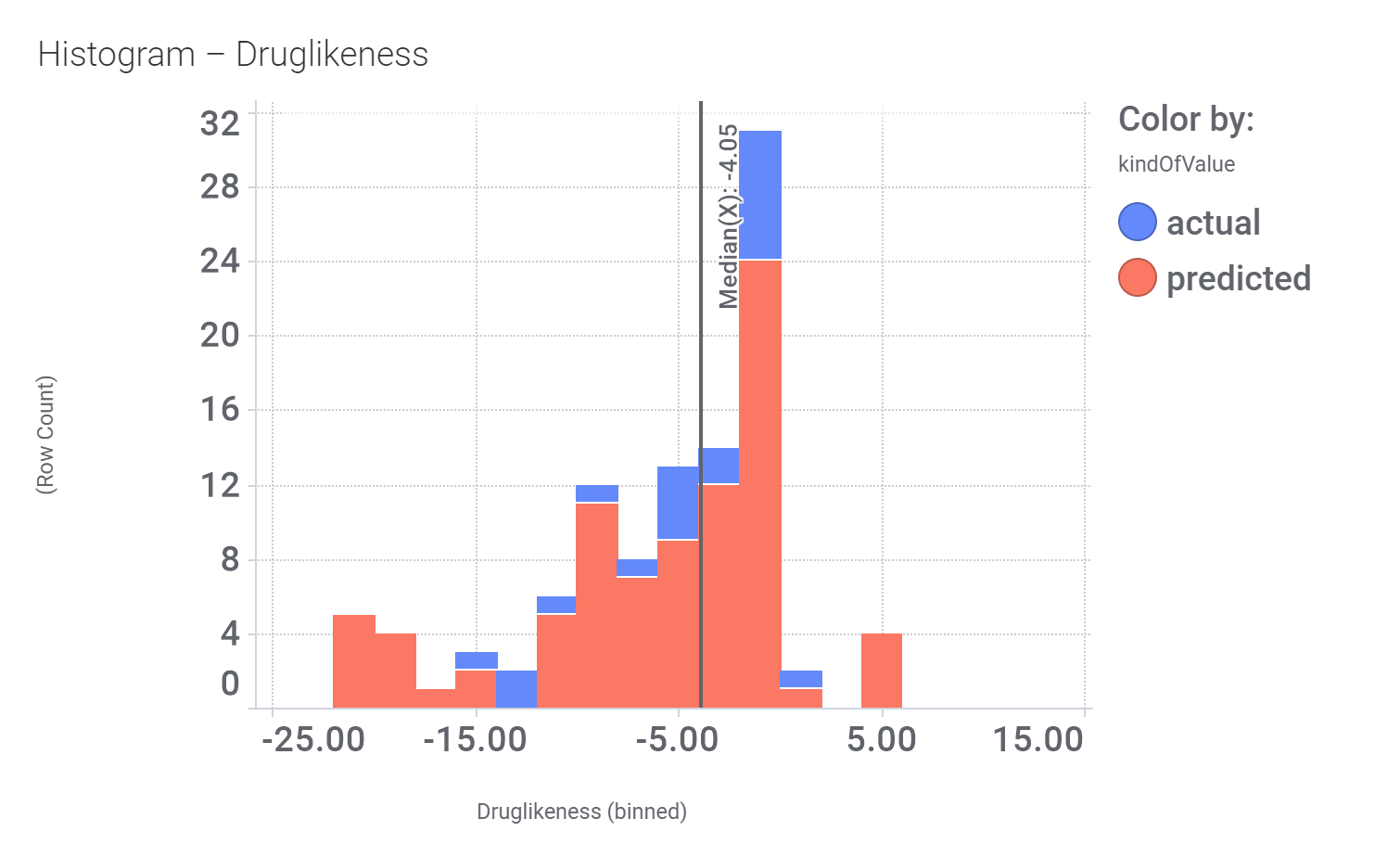
| Druglikeness | -9.5226 |
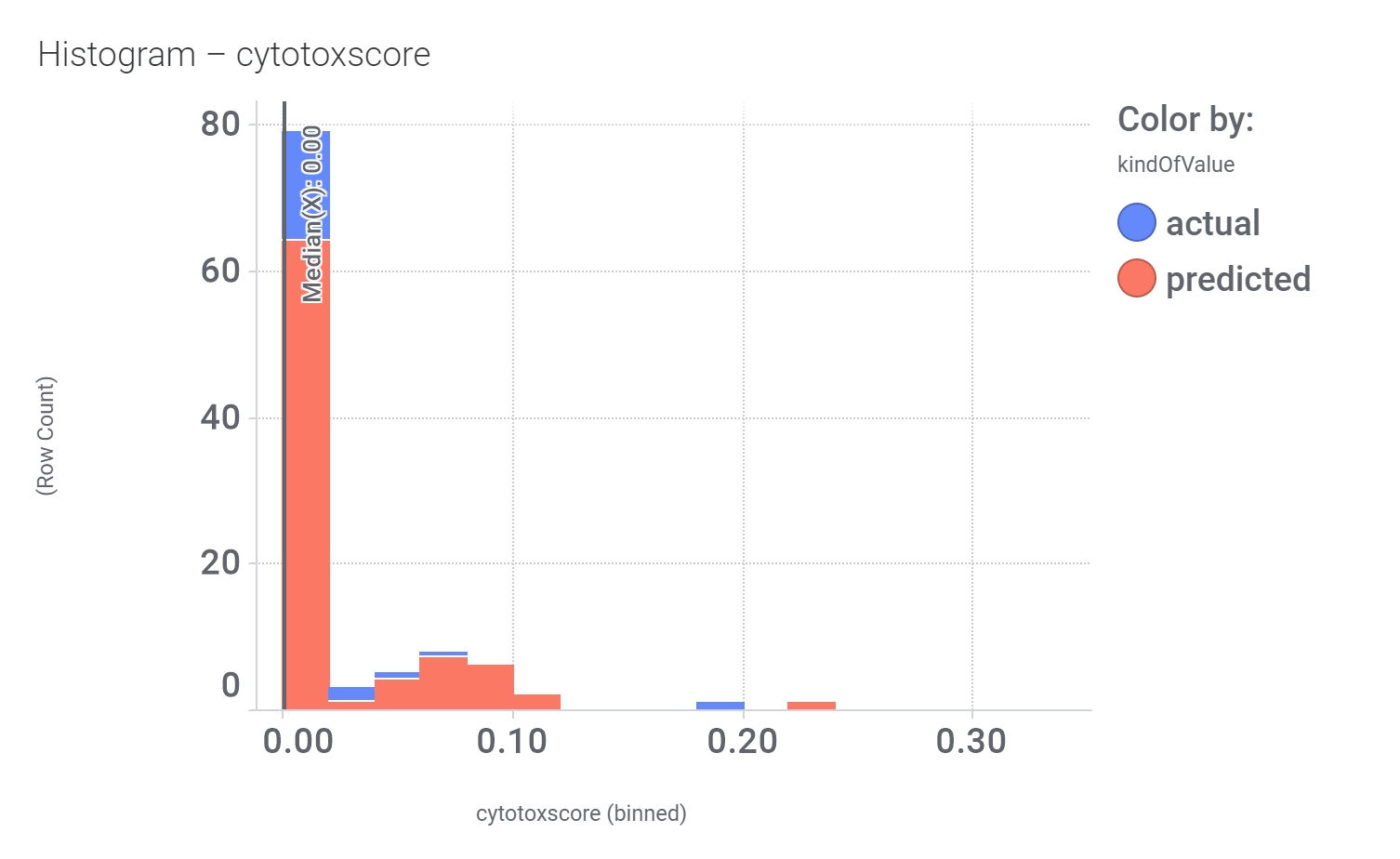
| cytotoxscore | 0 |
Literature references
Br J Pharmacol. 2022 Feb; 179(4): 511–525. doi: 10.1111/bph.15446
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
3.1.3. Pyrimidines
Using cell‐based and insulin secretion assays with rodent and human islets to screen a small library of compounds, generated through a three‐dimensional pharmacophore model, followed by chemical modification, Sloop et al. (2010) at Eli Lilly and Company reported a series of pyrimidine‐based compounds, represented by compound 10 (Compound B, or BETP) (Figure 3, [10]), that activate GLP‐1 receptors and stimulate glucose‐dependent insulin secretion. Similar to the quinoxalines, these compounds exhibit both agonistic and positive allosteric modulating effects on GLP‐1 receptors. BETP alone induced GLP‐1 receptor‐mediated cAMP signalling in HEK293 cells, leading to increased insulin secretion from rodent and human islets in a dose‐dependent manner. Combined with endogenous GLP‐1 peptide, BETP did not compete with 125I‐GLP‐1 but instead acted in an additive manner to induce both cAMP production and insulin secretion, suggesting BETP binds in an allosteric site and can function as a PAM. Consistent with these results, the activity of BETP was not blocked by the GLP‐1 competitive antagonist exendin‐4(9–39), and BETP can activate even the ECD truncated GLP‐1 receptor. In vivo studies show that BETP can stimulate insulin secretion in Sprague–Dawley rats.
In a subsequent study, the potential modulating effects of BETP on oxyntomodulin were examined (Willard, Wootten, et al., 2012). In vitro studies were carried out using a heterologous system consisting of HEK293 cells expressing human GLP‐1 receptors, BETP potentiated oxyntomodulin‐induced GLP‐1 receptor signalling and increased the binding affinity of oxyntomodulin for GLP‐1 receptors. BETP also enhances the activation of the Gs protein. In vivo studies by performing an IVGTT in Wistar rats indicated that BETP enhanced oxyntomodulin‐stimulated insulin secretion. In line with these observations, BETP induced biased signalling at oxyntomodulin‐mediated GLP‐1 receptors by selectively enhancing cAMP accumulation over other pathways including Ca2+ mobilization, pERK1/2 or β‐arrestin recruitment. In several following studies, the probe dependence of BETP in the presence of truncated GLP‐1(7–36)NH2 and GLP‐1(9–36)NH2 was further confirmed (Bueno et al., 2016; Li et al., 2012).
In another study, the mode of action of BETP was compared with compound 2, and clear differences were observed (Cheong et al., 2012). Both compounds can function as the GLP‐1 receptor agonist and independently activate human GLP‐1 receptors. However, they exert different probe dependence in the presence of full‐length GLP‐1, GLP‐1(9–36) or exenatide. Compound 2 shows an additive effect but did not increase the maximum efficacy when combined with full‐length GLP‐1, whereas BETP increases the maximum efficacy of GLP‐1 in a concentration‐dependent manner. For Ca2+ influx, both compounds slowly increase Ca2+ influx. However, the response induced by BETP lasts longer than that of compound 2 and GLP‐1. A similar study has also confirmed that BETP and compound 2 display different probe dependence and induce distinctive biased signalling (Wootten et al., 2013). Given the evident structural differences between the pyrimidine series and the quinoxaline series which may result in different active conformations, their different action pattern is expected.
To further understand the mechanism of BETP on GLP‐1 receptor activation and signalling, Eng et al. (2013) have determined that BETP covalently modifies Cys 347 located in the third intracellular loop of GLP‐1 receptors and this Cys residue is critical for its allosteric effect (Figure 4). Another study showed that diverse PAMs of GLP‐1 receptors including BETP and compound 2, with their electrophilic nature, all activate the receptor via the same mechanism as BETP (Bueno et al., 2016). Specifically, compound 2 is proposed to be located orthogonally above helix 6 and form multiple interactions with residues at the interface of helices 5 and 6. This binding model was experimentally validated by mutagenesis studies (Song et al., 2017). As both BETP and compound 2 as well as DA‐15864 and several others PAMs, all covalently modify the C347 residue of GLP‐1 receptors (Bueno et al., 2016; Eng et al., 2013) and display poor pharmacokinetic properties, these factors have prevented their further clinical development (Willard, Bueno et al., 2012).
3.1.4. PhotoETP
Both compound 2 and BETP demonstrate several attractive features that may be beneficial to the mechanistic studies of allosteric regulation of GLP‐1 receptors. For instance, both compounds showed probe dependence and both can induce biased signalling of the GLP‐1 receptor pathway in the presence of various GLP‐1 receptor peptides. To fine‐tune the activity of BETP, Broichhagen et al. (2016) designed an optically activated analogue of BETP (PhotoETP) in which the O‐benzyl group of BETP was replaced by an azobenzene group (compound 11) (Figure 3, [11]). This PhotoETP can be activated or inactivated by switching between the trans‐isomer and cis‐isomer by light. Although both the trans‐isomer and the cis‐isomer can augment insulin secretion in response to GLP‐1(7–36)NH2, only the trans‐isomer demonstrates modulating activity profile similar to BETP. In CHO cells expressing GLP‐1 receptors, only the trans‐isomer activates cAMP production in the presence of GLP‐1(9–36)NH2; in beta‐cell islets, only the trans‐isomer increases Ca2+ levels in GLP‐1(7–36)NH2‐induced signalling.
In a more recent report, the same group experimented with varying moieties of the base structures of BETP with restricted degrees of freedom in order to understand the effects of ligand conformation in allosteric binding of GLP‐1 receptors (Jones et al., 2017). Compounds incorporating a trans‐stilbene outperform those with cis‐stilbene, as well as BETP, in enhancing cAMP accumulation and Ca2+ influx.
However…
BETP and PhotoETP share stilbenoid and sulfinyl-trifluoromethyl-pyrimidine groups. Taken separately, there are >100 compounds showing similarity for either of the 2 substructures.
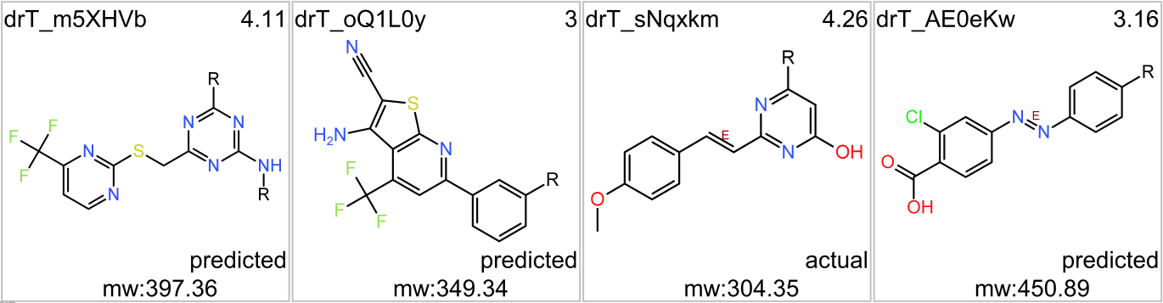
Here, some chosen exemplars among them.
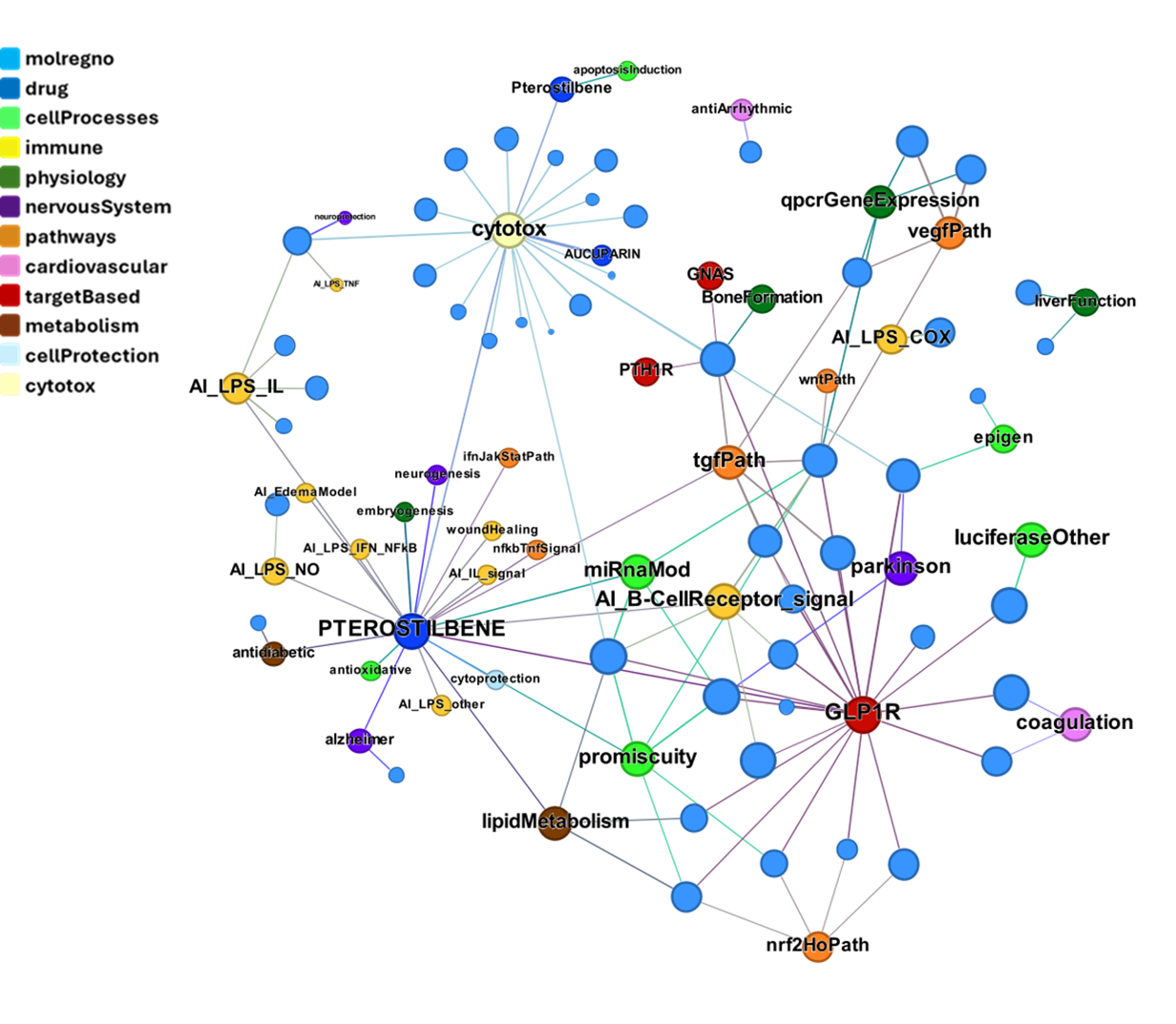
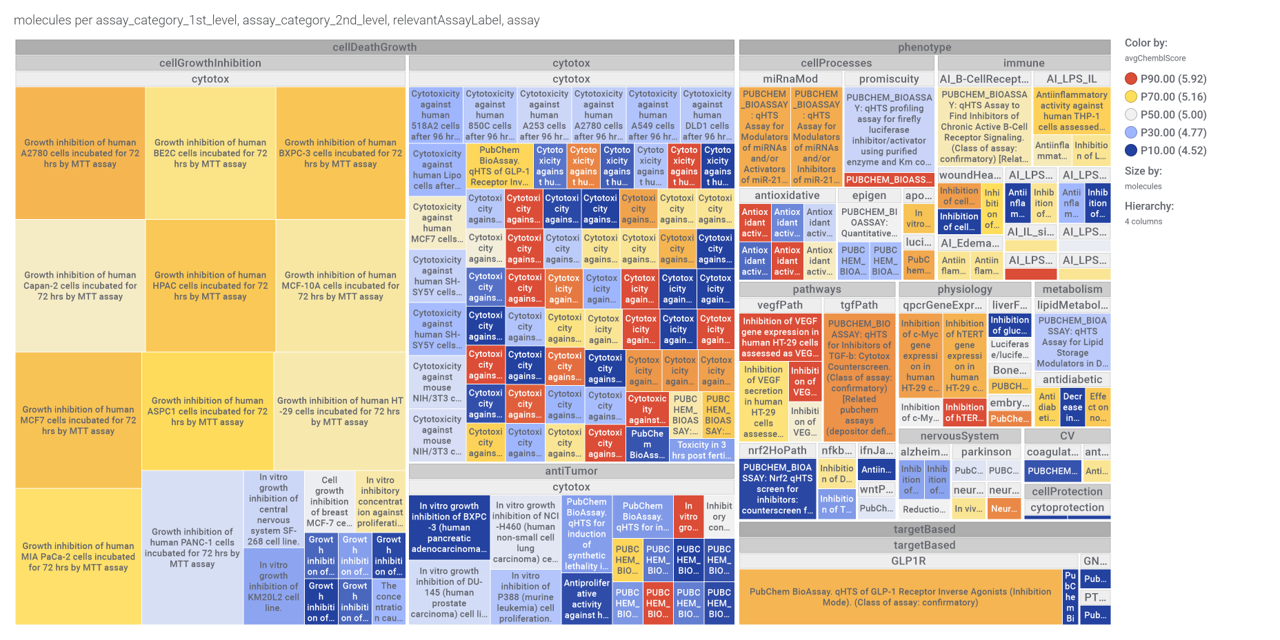
Let’s use a network graph to visualize the interactions of these compounds with relevant ChEMBL phenotypes.
And a treeMap to look at specific ChEMBL assays.
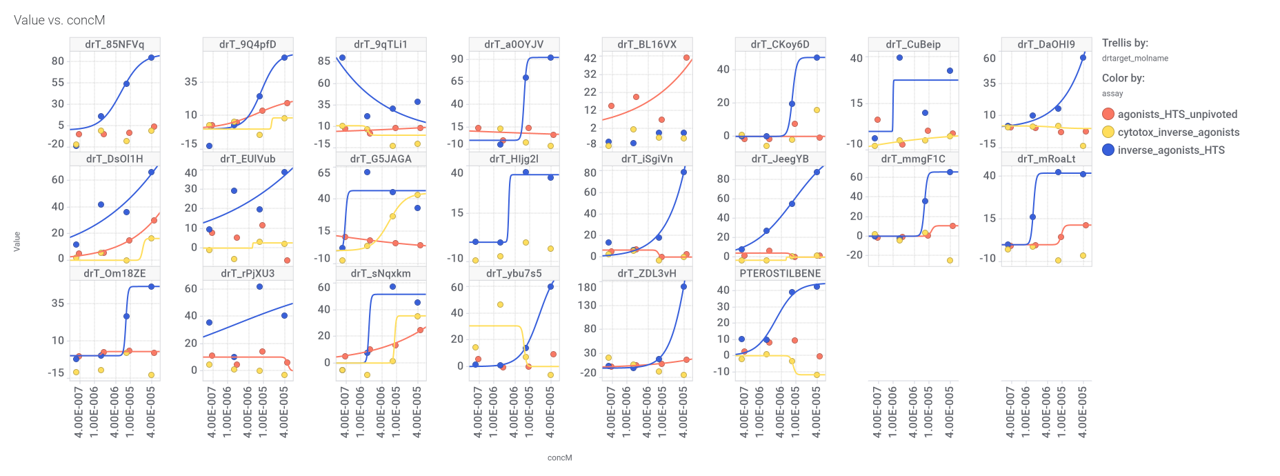
Some of these compounds show activity in the PubChem screens used to build the machine learning model.
Click below to examine distribution histograms for predicted GLP1R score, MW and developability properties.
Additional Literature references
ChemistryOpen. 2017 Aug; 6(4): 501–505. doi: 10.1002/open.201700062
Potent Prearranged Positive Allosteric Modulators of the Glucagon‐like Peptide‐1 Receptor
Dr. Ben J. Jones, 1 Dr. Rosario Scopelliti, 2 Dr. Alejandra Tomas, 3 Prof. Dr. Stephen R. Bloom, 1 Prof. Dr. David J. Hodson, 4 , 5 and Dr. Johannes Broichhagen 2 , 6
Abstract
Drugs that allosterically modulate G protein‐coupled receptor (GPCR) activity display higher specificity and may improve disease treatment. However, the rational design of compounds that target the allosteric site is difficult, as conformations required for receptor activation are poorly understood. Guided by photopharmacology, a set of prearranged positive allosteric modulators (PAMs) with restricted degrees of freedom was designed and tested against the glucagon‐like peptide‐1 receptor (GLP‐1R), a GPCR involved in glucose homeostasis. Compounds incorporating a trans‐stilbene comprehensively outperformed those with a cis‐stilbene, as well as the benchmark BETP, as GLP‐1R PAMs. We also identified major effects of ligand conformation on GLP‐1R binding kinetics and signal bias. Thus, we describe a photopharmacology‐directed approach for rational drug design, and introduce a new class of stilbene‐containing PAM for the specific regulation of GPCR activity.
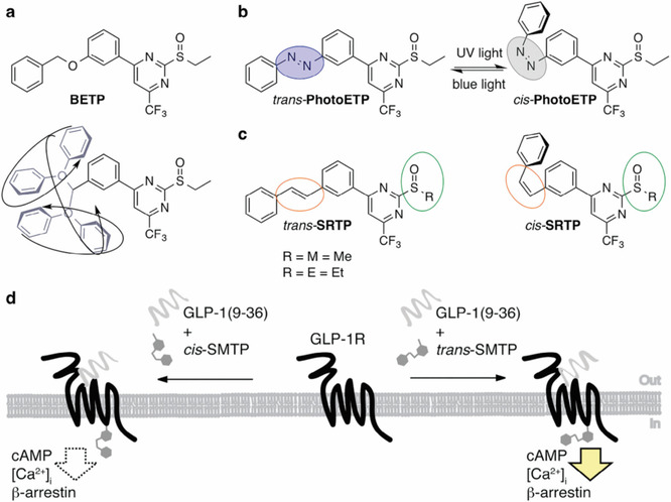
Logic and design of prearranged GLP-1R positive allosteric modulators. a) BETP possesses an O-benzyl ether that can freely rotate and adopt various conformations, occupying a large space, as denoted in the lower drawing. b) PhotoETP3 endows GLP-1R with light sensitivity when the azobenzene is locked in its active trans-state under blue light illumination (blue circle), which is interchangeable with UV light to its inactive cis-state (gray circle). c) Novel stilbene congeners are constantly locked (orange circle) in their respective state and do not exhibit photostationary states. The functional group for displacement can be a methyl (Me) or ethyl (Et) sulfoxide (green circle), giving pure trans- and cis-isomers of the prearranged PAMs SMTP and SETP. d) Schematic representation of GLP-1R activation with GLP-1(9–36)NH2 in the presence of either trans- or cis-SMTP.
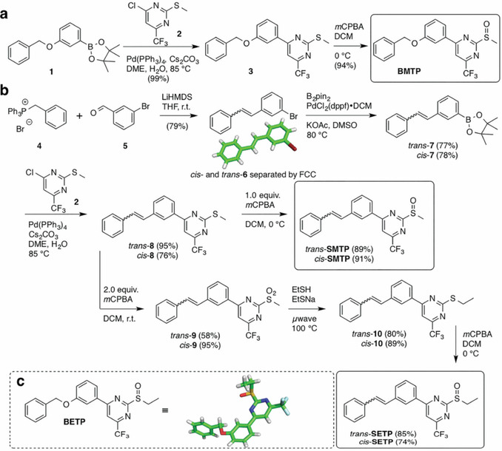
Synthesis of GLP-1R prearranged PAMs. a) BMTP is obtained after a two-step synthetic sequence from commercially available boronic ester 1 and chloropyrimidine 2 after Pd cross-coupling and subsequent monooxidation. b) Wittig transformation gives access to trans- and cis-stilbenes that can be separated by FCC and independently processed towards thioether 8. trans- and cis-SMTP are obtained as the monooxidized products from 8, whereas bisoxidation, aromatic substitution, and monooxidation yield the prearranged positive allosteric modulators trans- and cis-SETP. c) Crystal structure of BETP.
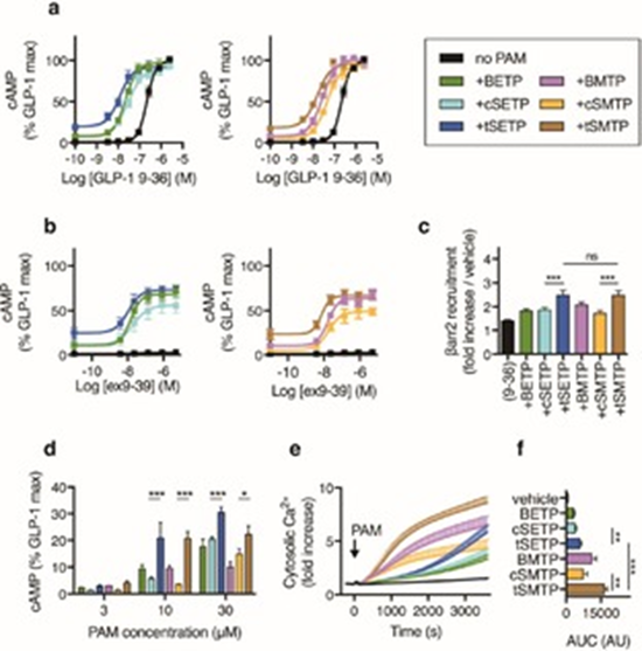
Prearranged PAMs potently enhance GLP-1R signaling. a) Allosteric enhancement of GLP-1(9–36)NH2 (“9–36”) cAMP responses (30 min incubation; n=4) (4-parameter logistic fit shown). b) As for (a), but with exendin(9–39) (ex9-39) (n=5). c) Allosteric enhancement of 10 μμ GLP-1(9–36)NH2-induced β-arrestin2 (βarr2) recruitment (30 min incubation; n=5). d) cAMP responses to indicated PAM or prearranged PAM concentration in the absence of orthosteric ligand (30 min incubation; n=3). e) Cytosolic Ca2+ responses in Calcium 6 dye-loaded cells, expressed relative to baseline fluorescent signal (60 min incubation; n=7). f) Area under curve (AUC) determined from (e). *P<0.05, **P<0.01, ***P<0.001; one- or two-way randomized block ANOVA followed by either Sidak’s or Tukey’s post-hoc test. Values represent the mean + or ± SEM. Except where indicated, PAMs or prearranged PAMs were applied at 10 μμ.