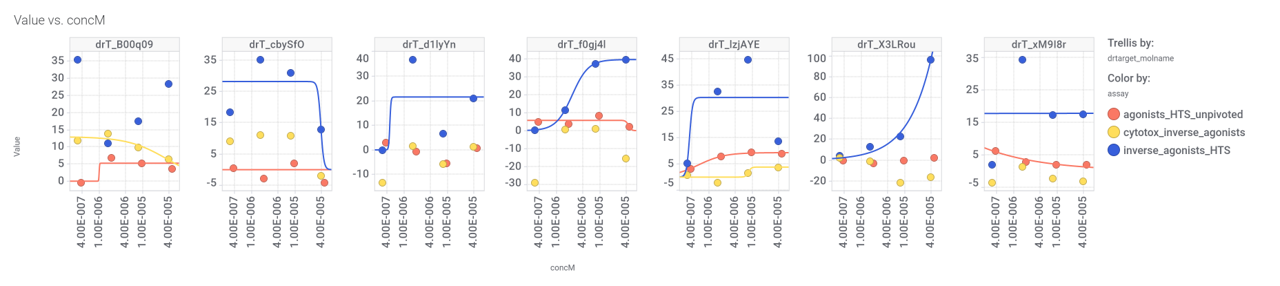
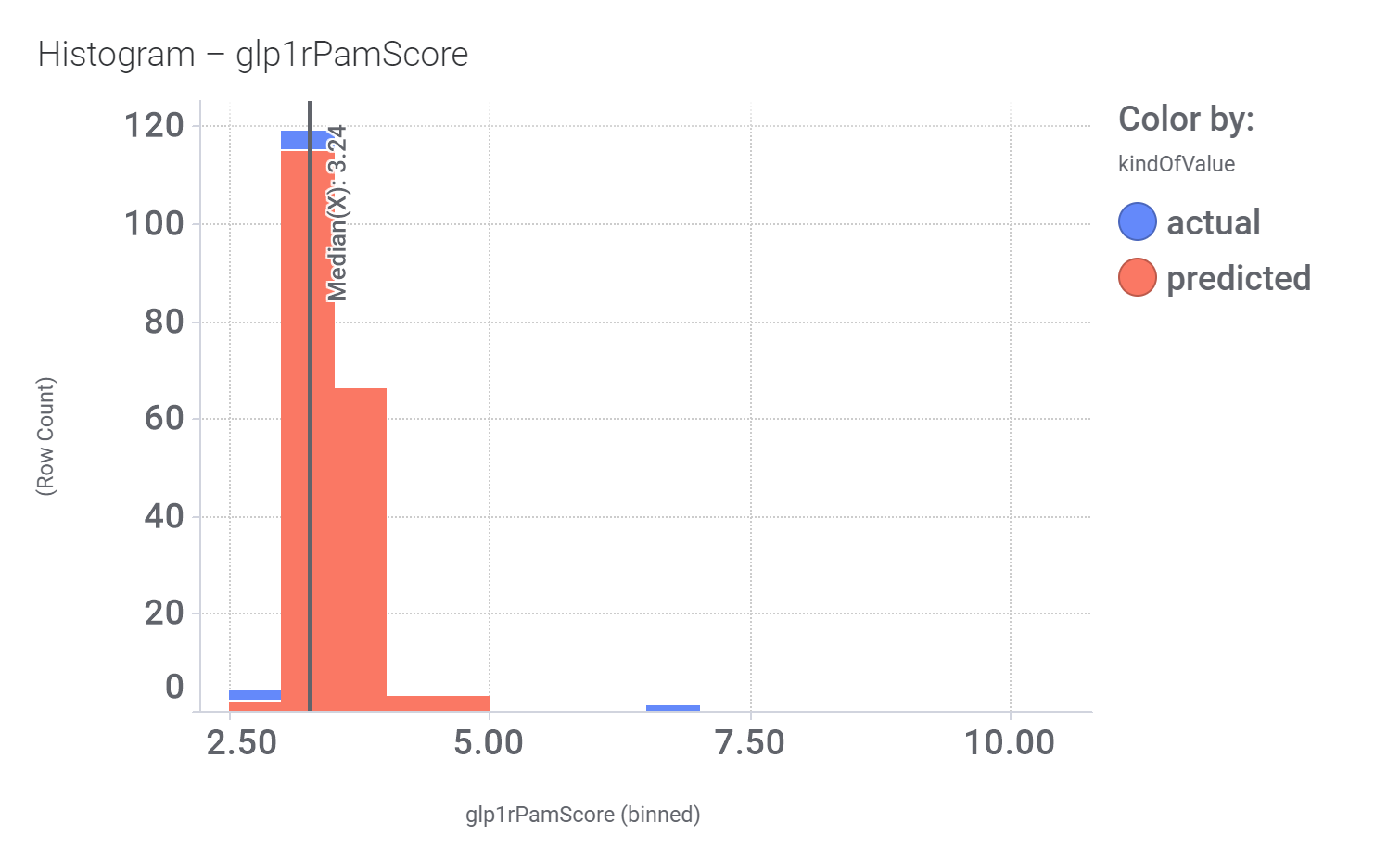
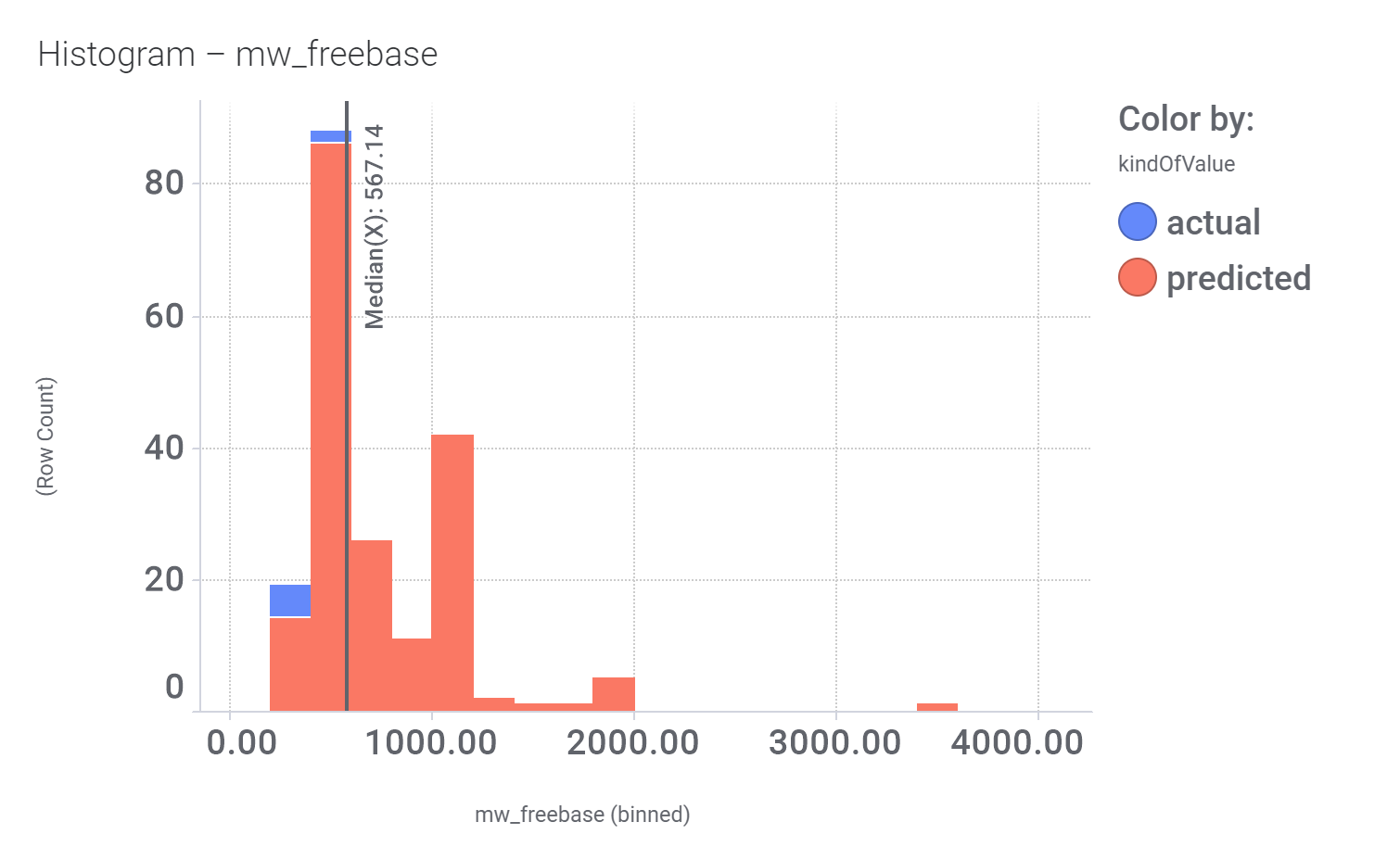
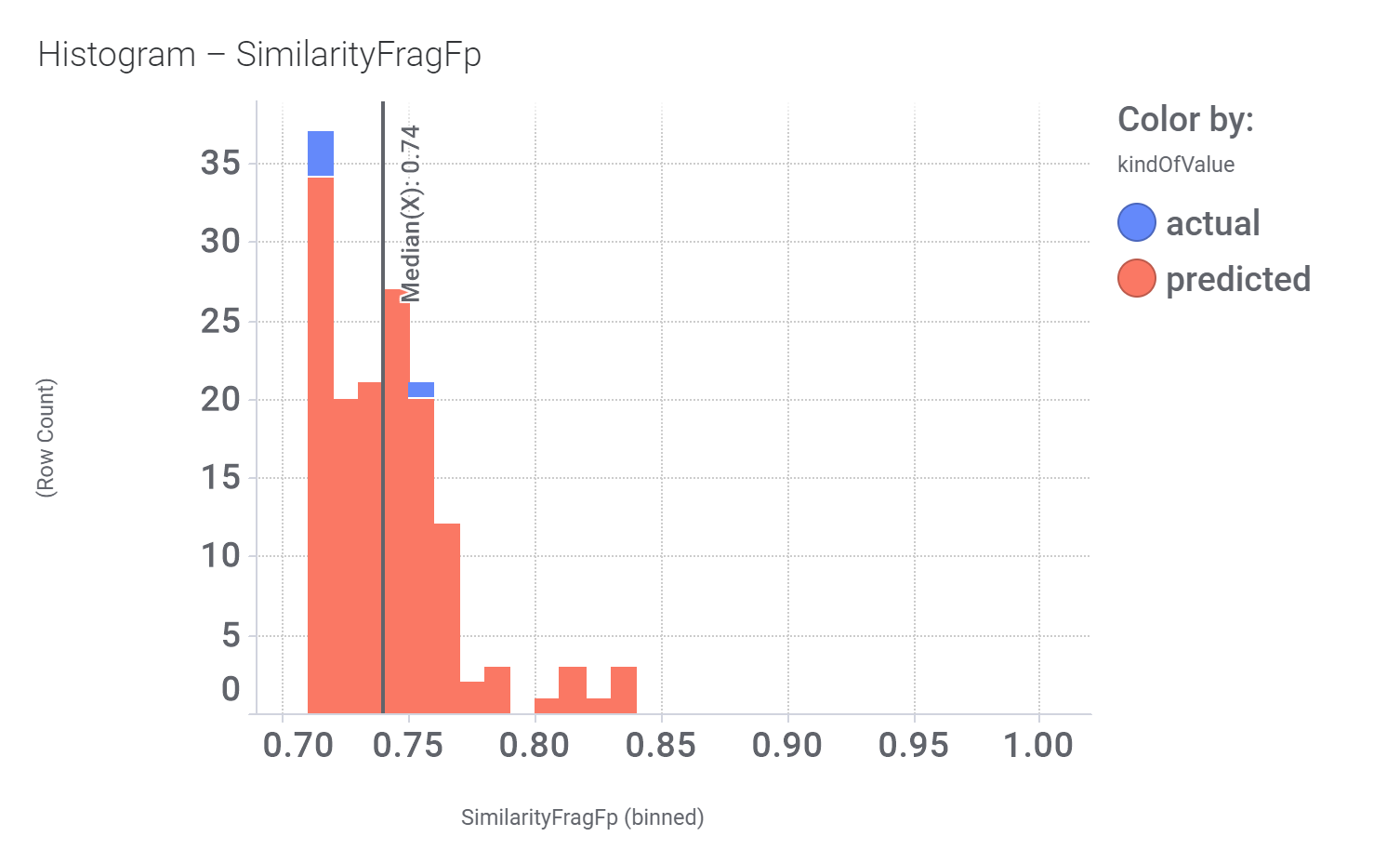
This molecule, isolated from fenugreek (Trigonella foenum‐graecum) shows weak similarity with 196 predicted GLP1R PAM active compounds. Here, some exemplars.
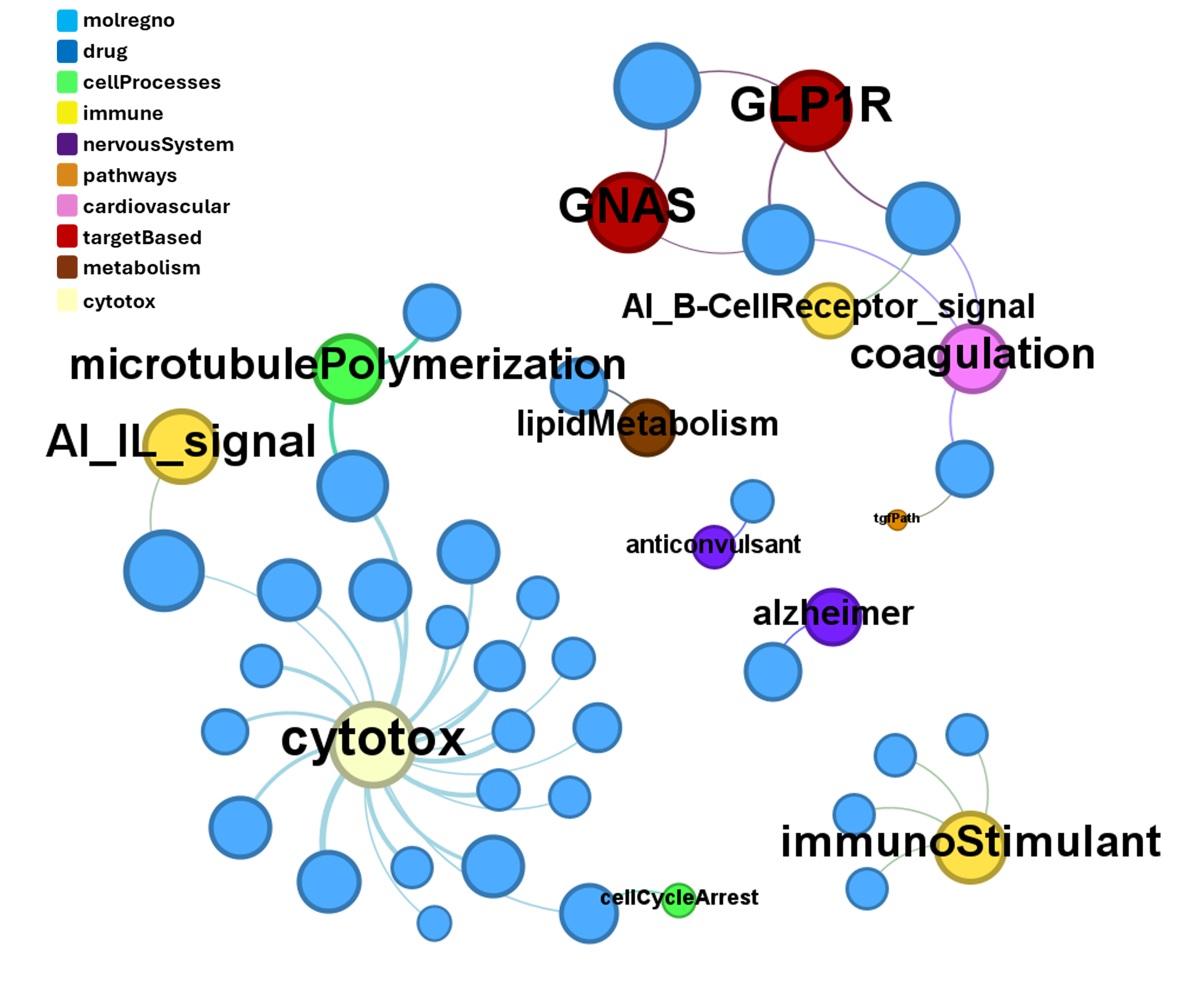
Their interaction map at relevant ChEMBL phenotypes can be visualized with a network graph.
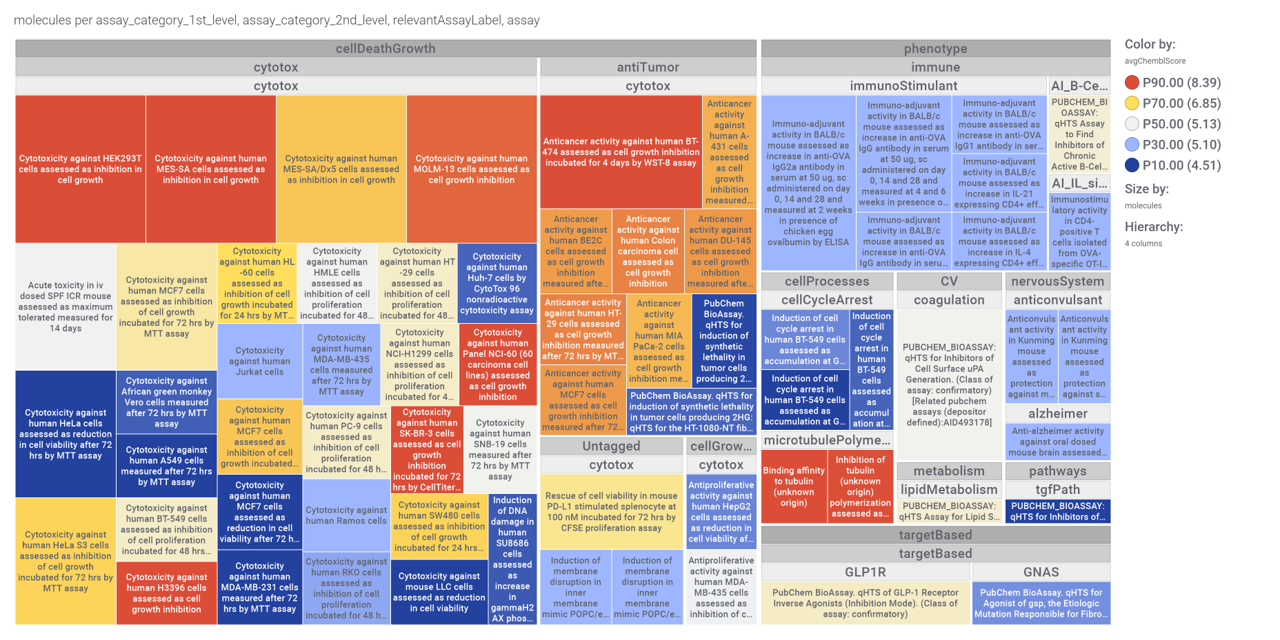
ChEMBL assays associated to these phenotypes are specified in this treeMap.
Few of these seem to have weak activity at PChem screens.
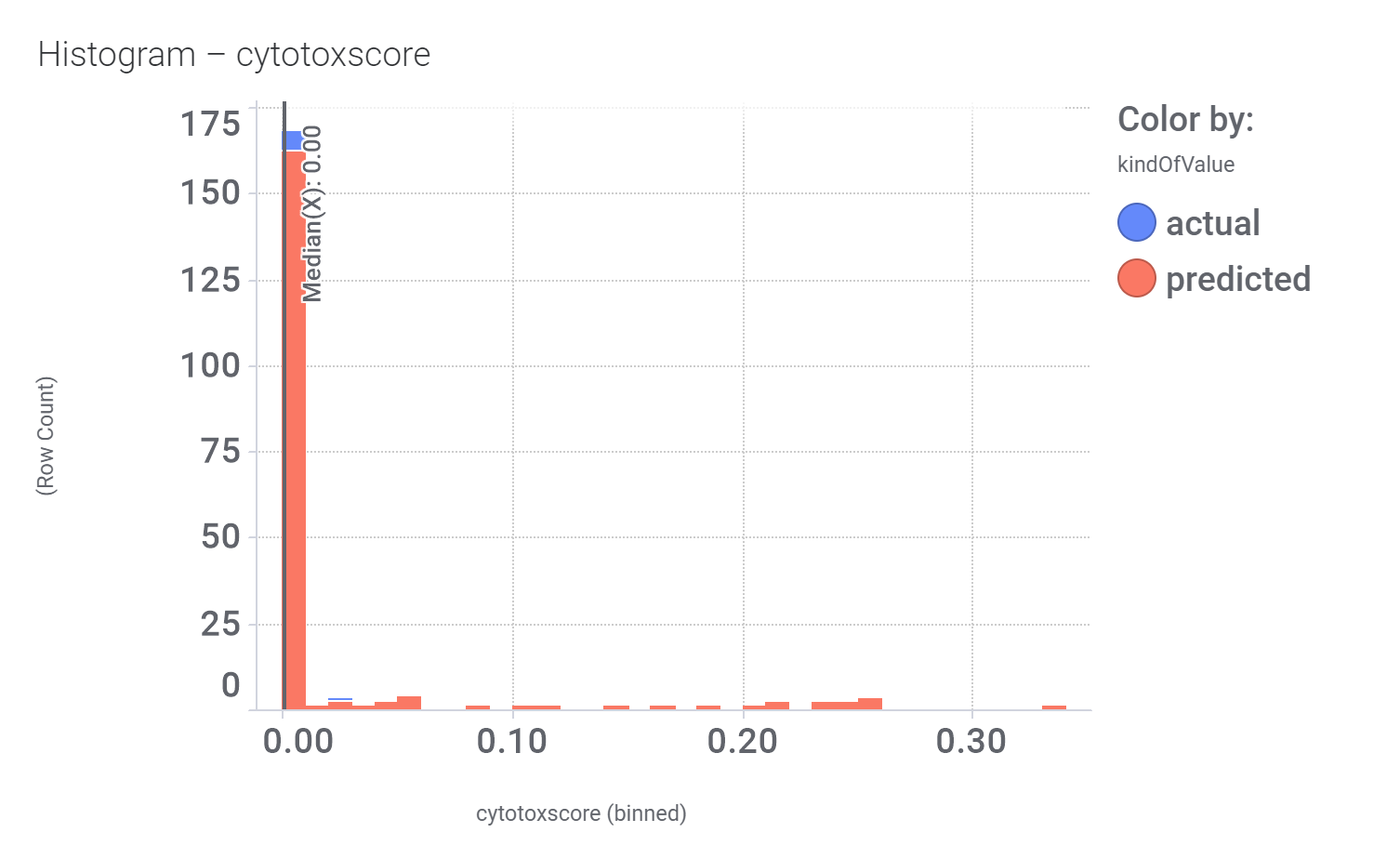
Here, their bio-physicochemical and developability properties.
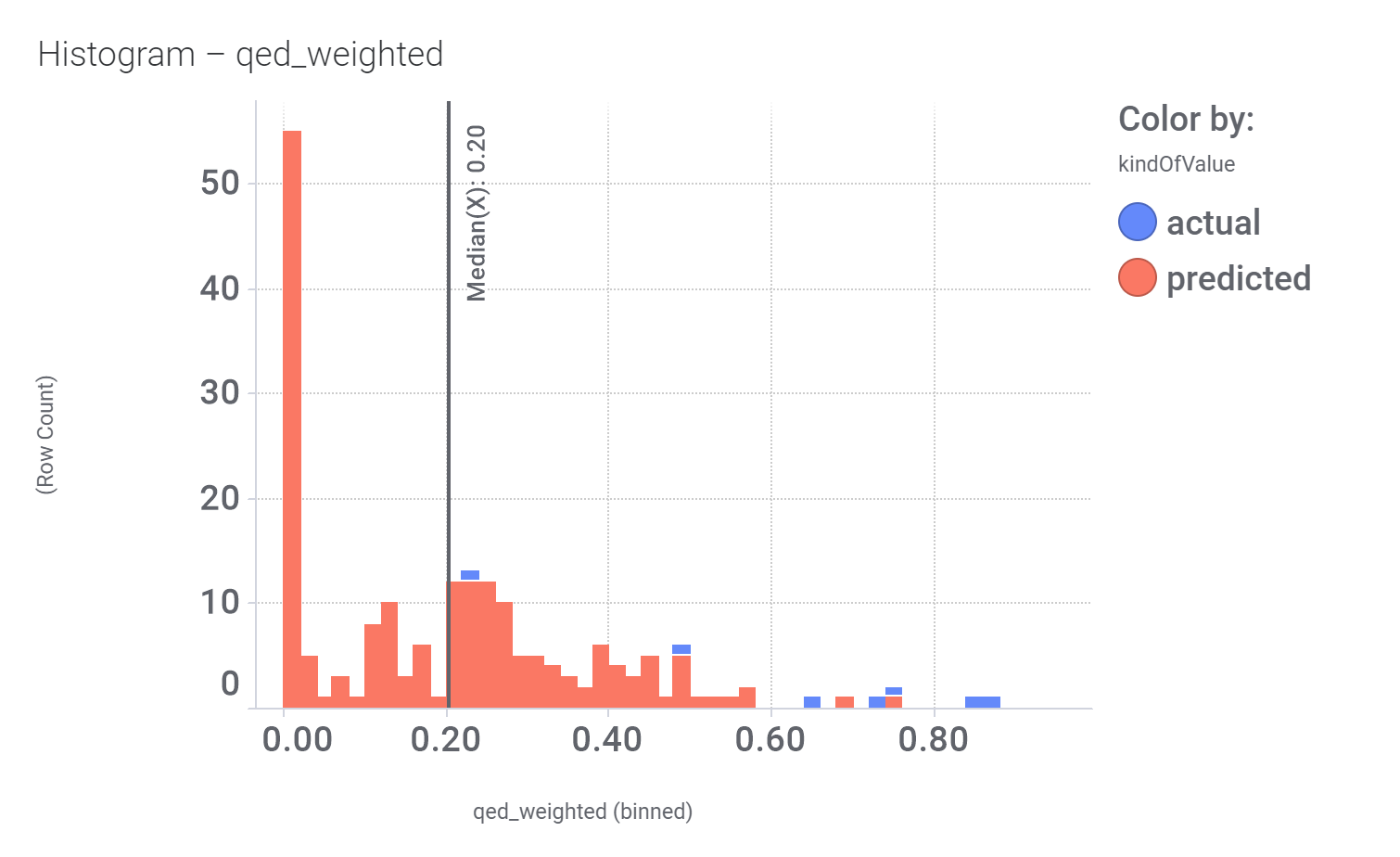
Druglikeness estimated by QedWeighted
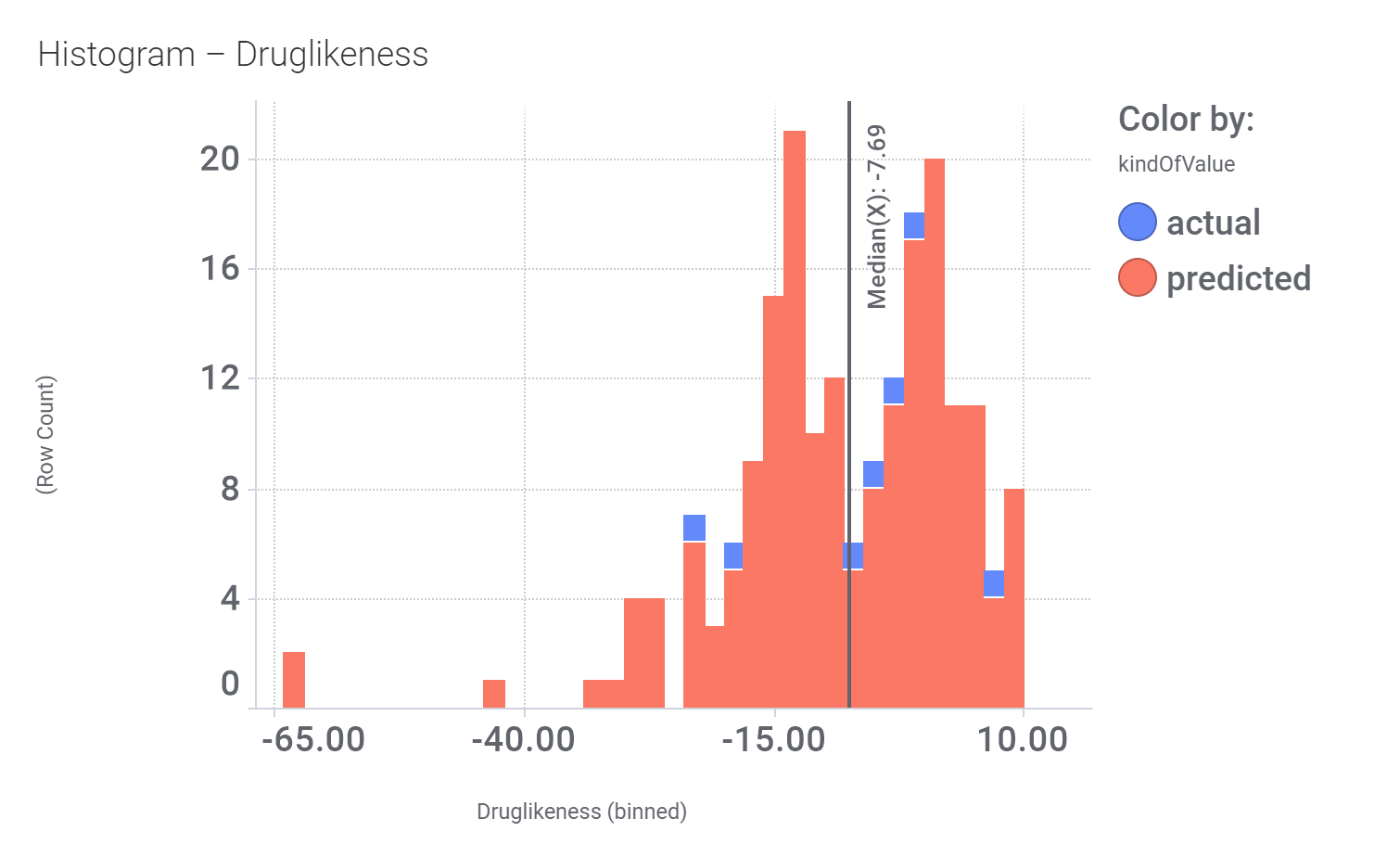
Druglikeness estimated by DataWarrior
Literature support.
Br J Pharmacol. 2022 Feb; 179(4): 511–525. doi: 10.1111/bph.15446
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
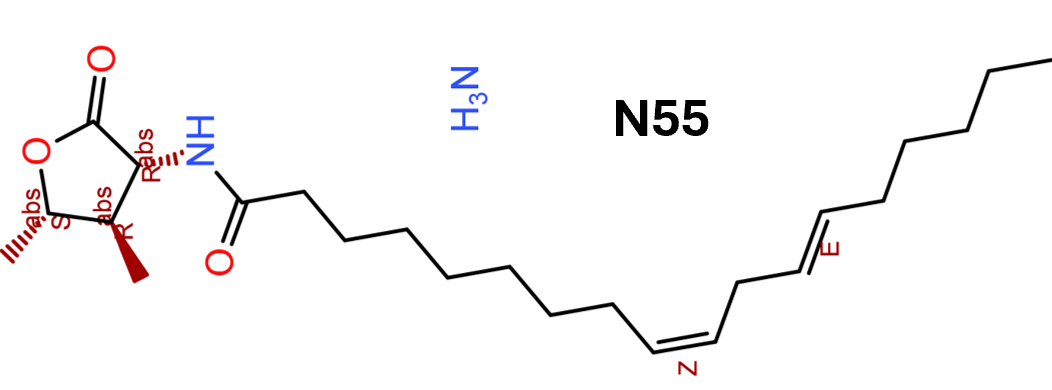
3.1.7. Compound N55
Fenugreek (Trigonella foenum‐graecum) seeds have been observed to reduce glucose and glycated haemoglobin levels, thereby alleviate some effects of Type 2 diabetes. By screening extracts from fenugreek seeds using an intracellular cAMP biosensor and GLP‐1 receptor endocytosis assays, King et al. (2015) found that ethanol extracts enhanced GLP‐1 signalling. Further purification identified an active compound 13 (N55) (Figure 3, [13]), which has a N‐linoleoyl‐2‐amino‐γ‐butyrolactone structure. In vitro studies show that N55 promotes GLP‐1‐dependent cAMP accumulation and GLP‐1 receptor endocytosis in a dose‐dependent manner. N55 also exhibits strong probe dependence in that it specifically enhances activity of GLP‐1(7–36)NH2 but not GIP or exendin‐4.
What is most interesting about compound N55 is its unique mechanism. GLP‐1 is known to bind to signal‐enhanced oleoylethanolamide (OEA) or 2‐oleoylglycerol but not to stearoylethanolamide (SEA) (Cheng et al., 2015). To understand the mechanism of N55, competition‐binding experiments were performed to examine the effect of increasing N55 concentrations on the binding of [3H] OEA to GLP‐1. As the concentration of N55 increased, binding of OEA gradually decreased. This was further confirmed by trypsin cleavage experiments. When the trypsin cleavage reactions were carried out in the presence of 77‐μM N55, the residual GLP‐1 activity was dramatically reduced compared with that without N55. These experiments suggest that rather than binding to an allosteric site on GLP‐1 receptors, like other known PAMs, N55 binds to GLP‐1(7–36)NH2 directly. The binding of N55 to GLP‐1 may induce conformational change in GLP‐1(7–36)NH2, thus slowing its cleavage and digestion by trypsin enzymes. Hence, N55 represents a new class of PAMs of GLP‐1 receptors and targeting GLP‐1 peptides could be a viable approach to modulation of GLP‐1 receptor activity.
J Biol Chem. 2015 Oct 23; 290(43): 26235–26248. doi: 10.1074/jbc.M115.672097. PMCID: PMC4646272. PMID: 26336108
Isolation of Positive Modulator of Glucagon-like Peptide-1 Signaling from Trigonella foenum-graecum (Fenugreek) Seed*
Klim King,‡,1 Nai-Pin Lin,§ Yu-Hong Cheng,‡ Gao-Hui Chen,¶ and Rong-Jie Chein§,2
Author information Article notes Copyright and License information PMC Disclaimer
Background: Fenugreek is an edible plant used to treat diabetes and other disorders.
Results: We isolated a new structure in fenugreek that enhances glucagon-like peptide-1 (GLP-1) potency.
Conclusion: The antidiabetic plant fenugreek is a rich source of GLP-1 activity-enhancing compounds.
Significance: GLP-1 peptide is a target for new drug screening and drug safety studies.
Abstract
The glucagon-like peptide-1 receptor (GLP-1R) is expressed in many tissues and has been implicated in diverse physiological functions, such as energy homeostasis and cognition. GLP-1 analogs are approved for treatment of type 2 diabetes and are undergoing clinical trials for other disorders, including neurodegenerative diseases. GLP-1 analog therapies maintain chronically high plasma levels of the analog and can lead to loss of spatiotemporal control of GLP-1R activation. To avoid adverse effects associated with current therapies, we characterized positive modulators of GLP-1R signaling. We screened extracts from edible plants using an intracellular cAMP biosensor and GLP-1R endocytosis assays. Ethanol extracts from fenugreek seeds enhanced GLP-1 signaling. These seeds have previously been found to reduce glucose and glycated hemoglobin levels in humans. An active compound (N55) with a new N-linoleoyl-2-amino-γ-butyrolactone structure was purified from fenugreek seeds. N55 promoted GLP-1-dependent cAMP production and GLP-1R endocytosis in a dose-dependent and saturable manner. N55 specifically enhanced GLP-1 potency more than 40-fold, but not that of exendin 4, to stimulate cAMP production. In contrast to the current allosteric modulators that bind to GLP-1R, N55 binds to GLP-1 peptide and facilitates trypsin-mediated GLP-1 inactivation. These findings identify a new class of modulators of GLP-1R signaling and suggest that GLP-1 might be a viable target for drug discovery. Our results also highlight a feasible approach for screening bioactive activity of plant extracts.
J Biol Chem. 2015 Jun 5; 290(23): 14302–14313. doi: 10.1074/jbc.M115.655662. PMCID: PMC4505500. PMID: 25903129
Modulation of Glucagon-like Peptide-1 (GLP-1) Potency by Endocannabinoid-like Lipids Represents a Novel Mode of Regulating GLP-1 Receptor Signaling*
Yu-Hong Cheng,‡ Mei-Shang Ho,§ Wei-Ting Huang,‡ Ying-Ting Chou,§ and Klim King‡,1
Background: Glucagon-like peptide-1 receptors (GLP-1Rs) are expressed in many tissues that are accessed only by a basal circulating level of GLP-1.
Results: GLP-1 potency is enhanced by specific endocannabinoid-like lipids.
Conclusion: Enhancing GLP-1 potency is a novel mechanism regulating GLP-1R signaling.
Significance: Spatiotemporal regulation of GLP-1R becomes possible at basal physiological levels of GLP-1 because endocannabinoid-like lipids are known to be physiologically regulated.
Abstract
Glucagon-like peptide-1 (GLP-1) analogs are approved for treatment of type 2 diabetes and are in clinical trials for disorders including neurodegenerative diseases. GLP-1 receptor (GLP-1R) is expressed in many peripheral and neuronal tissues and is activated by circulating GLP-1. Other than food intake, little is known about factors regulating GLP-1 secretion. Given a normally basal circulating level of GLP-1, knowledge of mechanisms regulating GLP-1R signaling, which has diverse functions in extrapancreatic tissues, remains elusive. In this study, we found that the potency of GLP-1, not exendin 4, is specifically enhanced by the endocannabinoid-like lipids oleoylethanolamide (OEA) and 2-oleoylglycerol but not by stearoylethanolamide (SEA) or palmitoylethanolamide. 9.2 μm OEA enhances the potency of GLP-1 in stimulating cAMP production by 10-fold but does not affect its receptor binding affinity. OEA and 2-oleoylglycerol, but not SEA, bind to GLP-1 in a dose-dependent and saturable manner. OEA but not SEA promoted GLP-1(7–36) amide to trypsin inactivation in a dose-dependent and saturable manner. Susceptibility of GLP-1(7–36) amide to trypsin inactivation is increased 40-fold upon binding to OEA but not to SEA. Our findings indicate that OEA binds to GLP-1(7–36) amide and enhances the potency that may result from a conformational change of the peptide. In conclusion, modulating potency of GLP-1 by physiologically regulated endocannabinoid-like lipids allows GLP-1R signaling to be regulated spatiotemporally at a constant basal GLP-1 level.