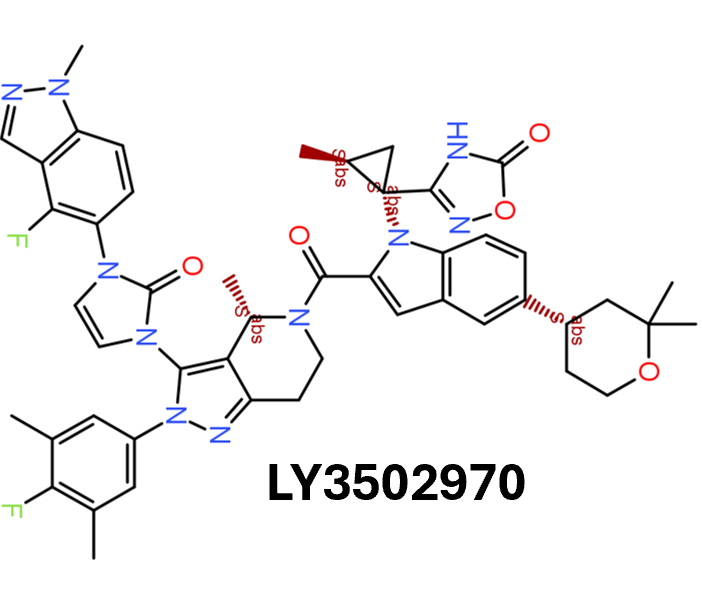
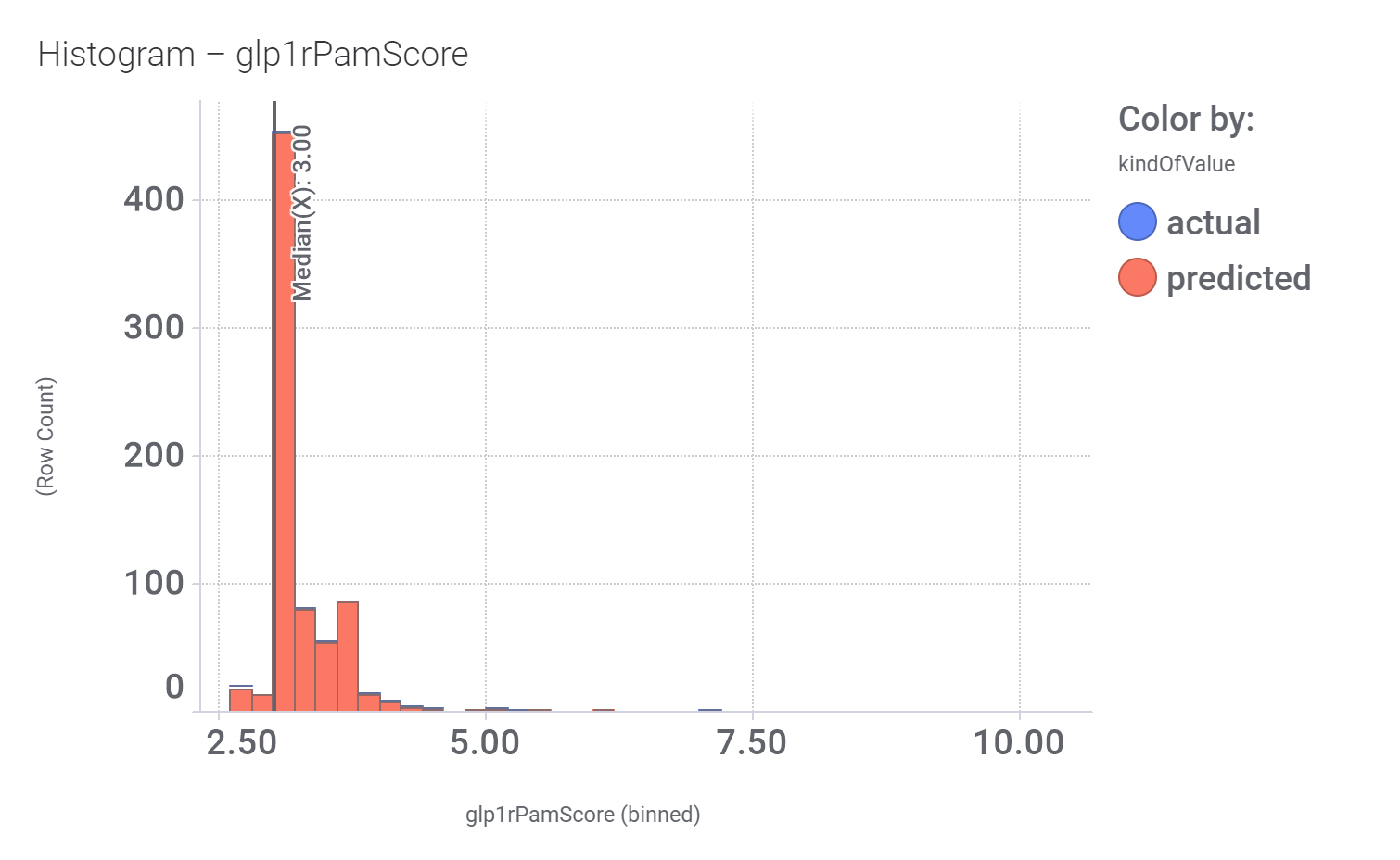
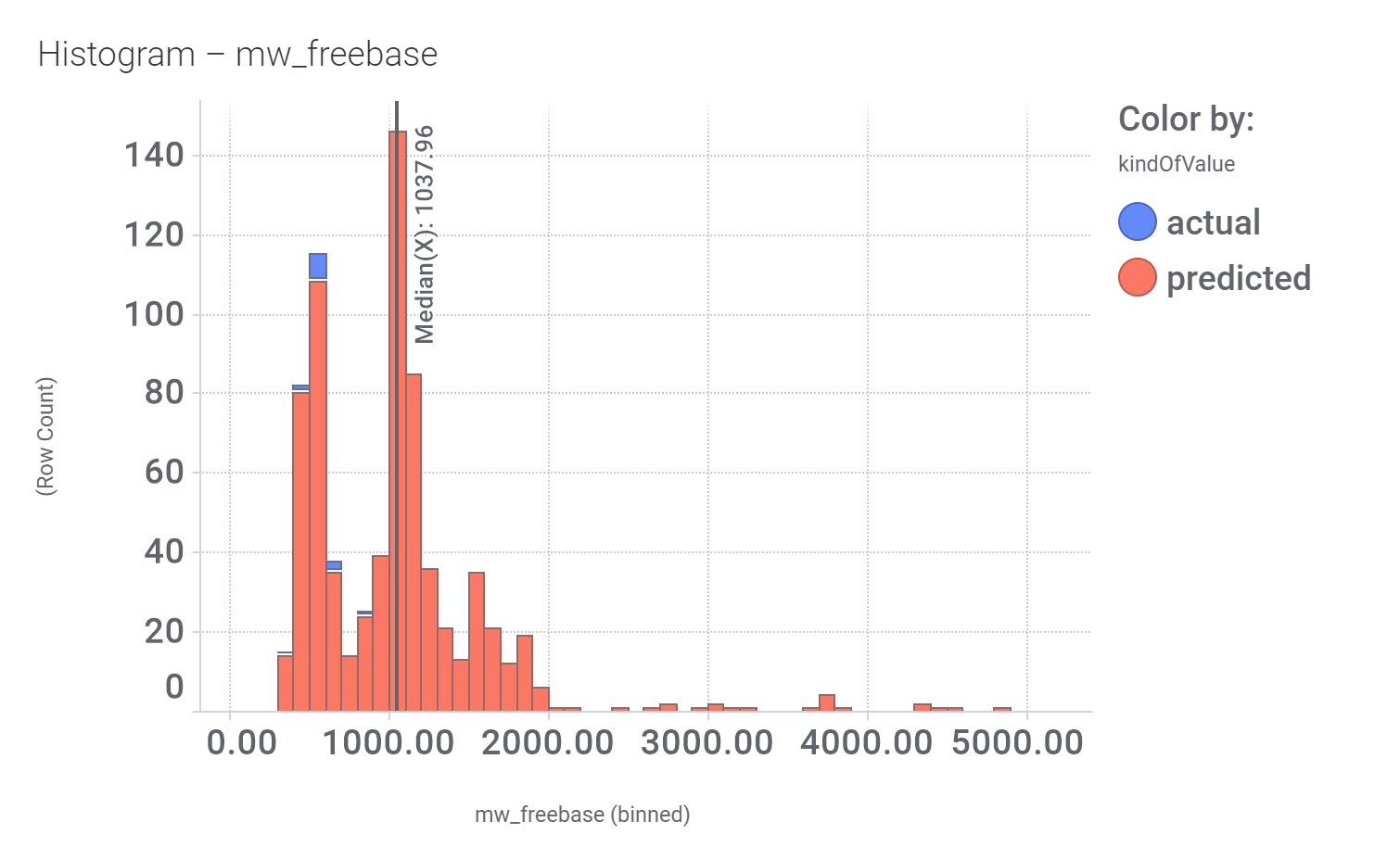
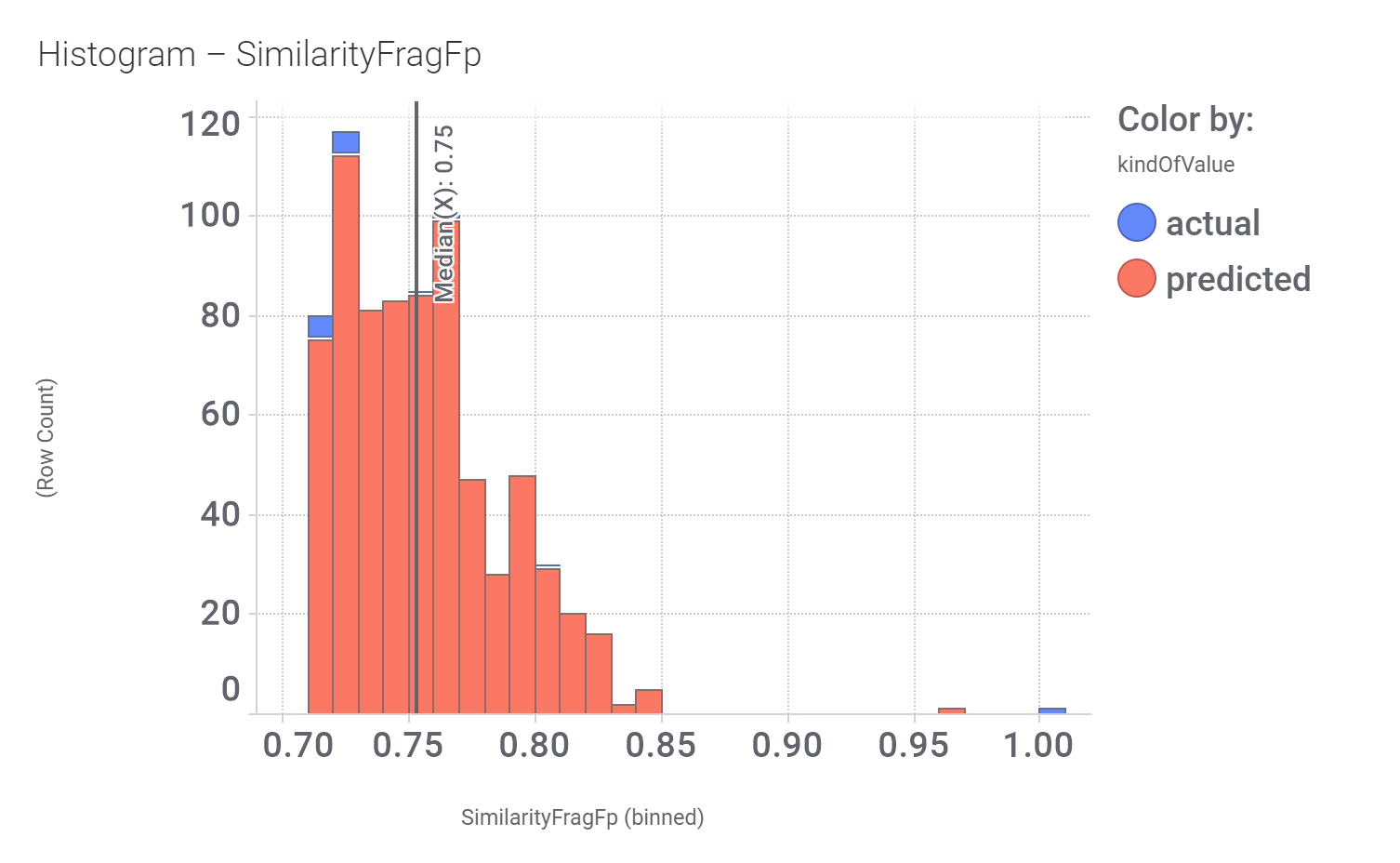
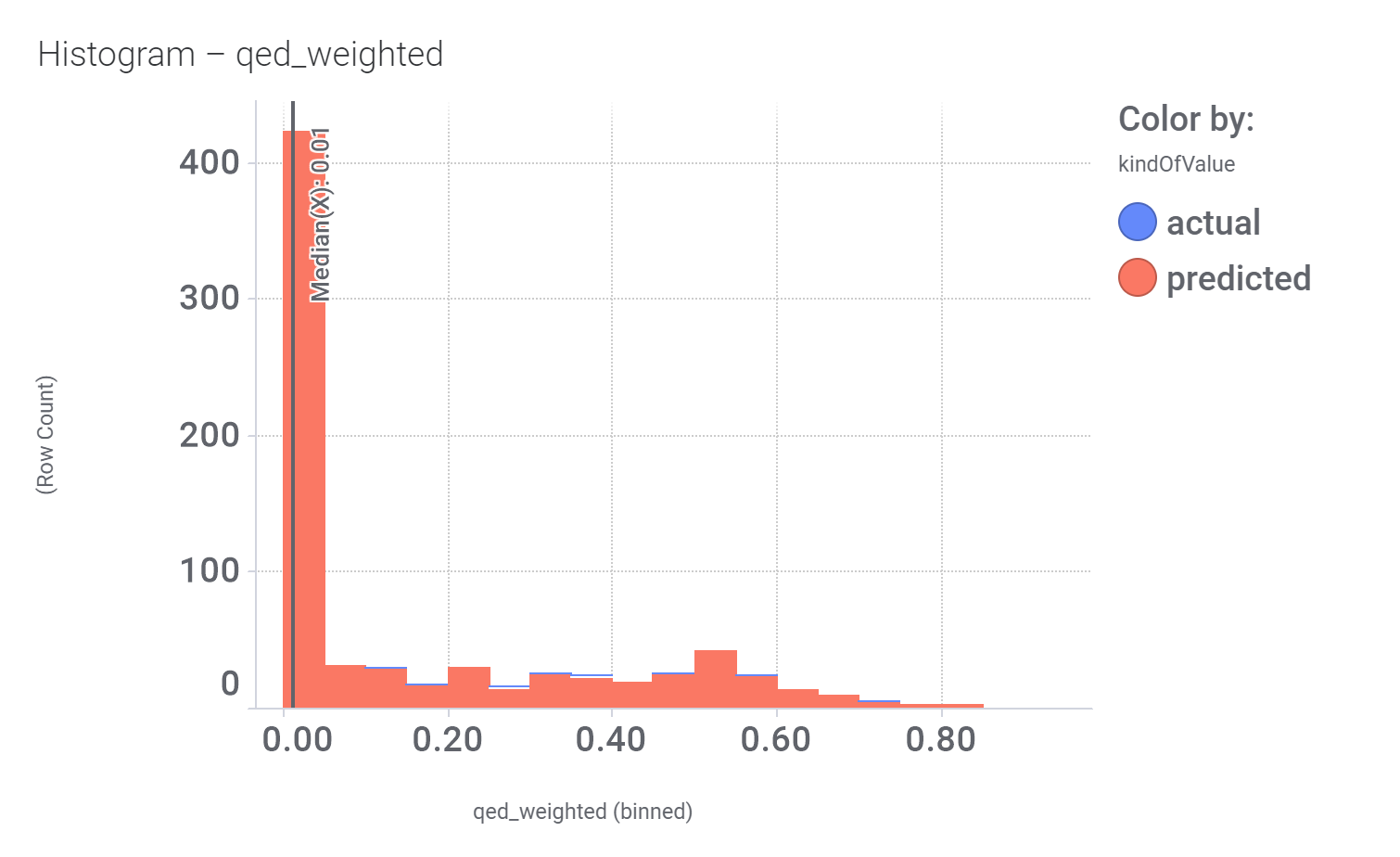
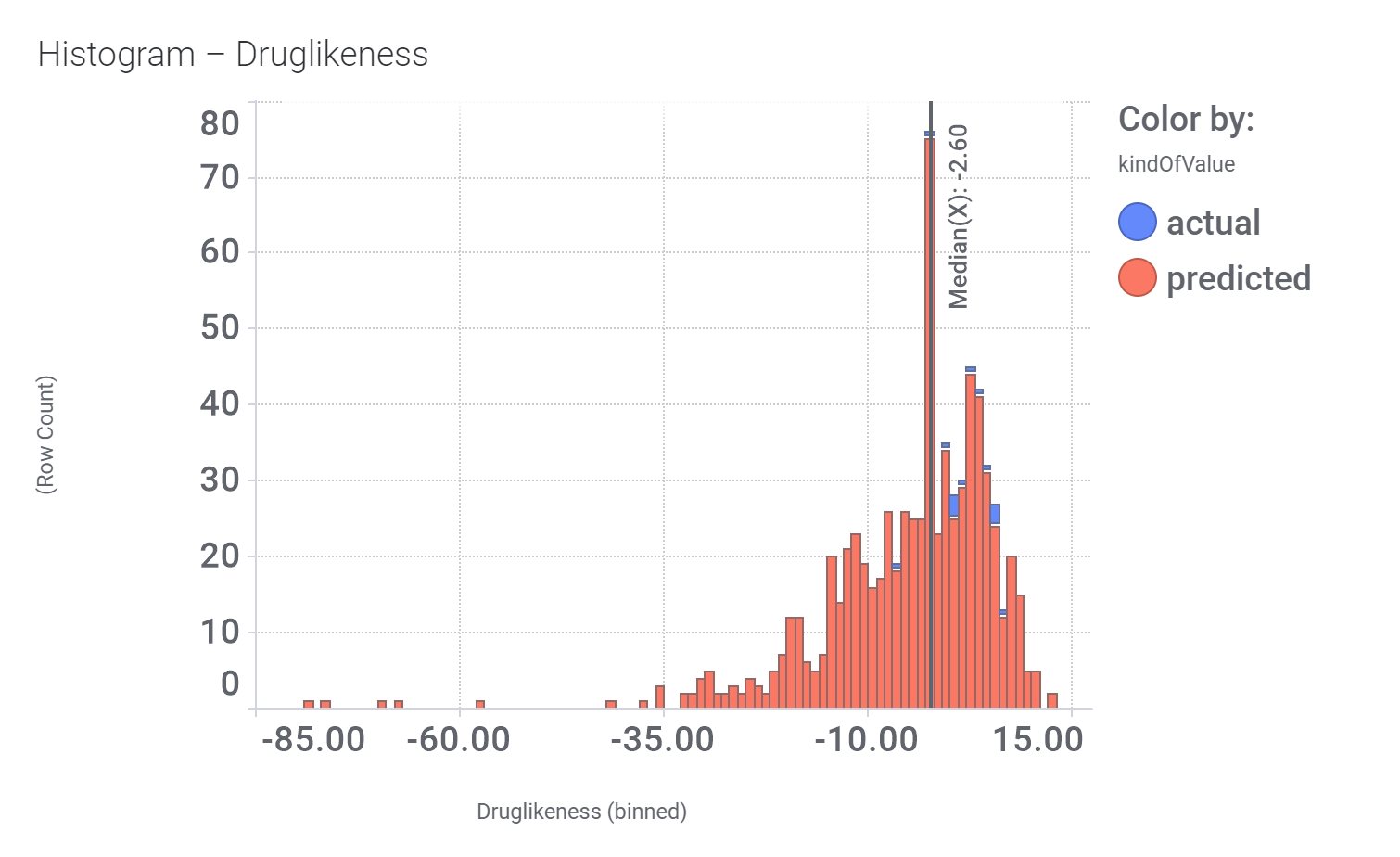
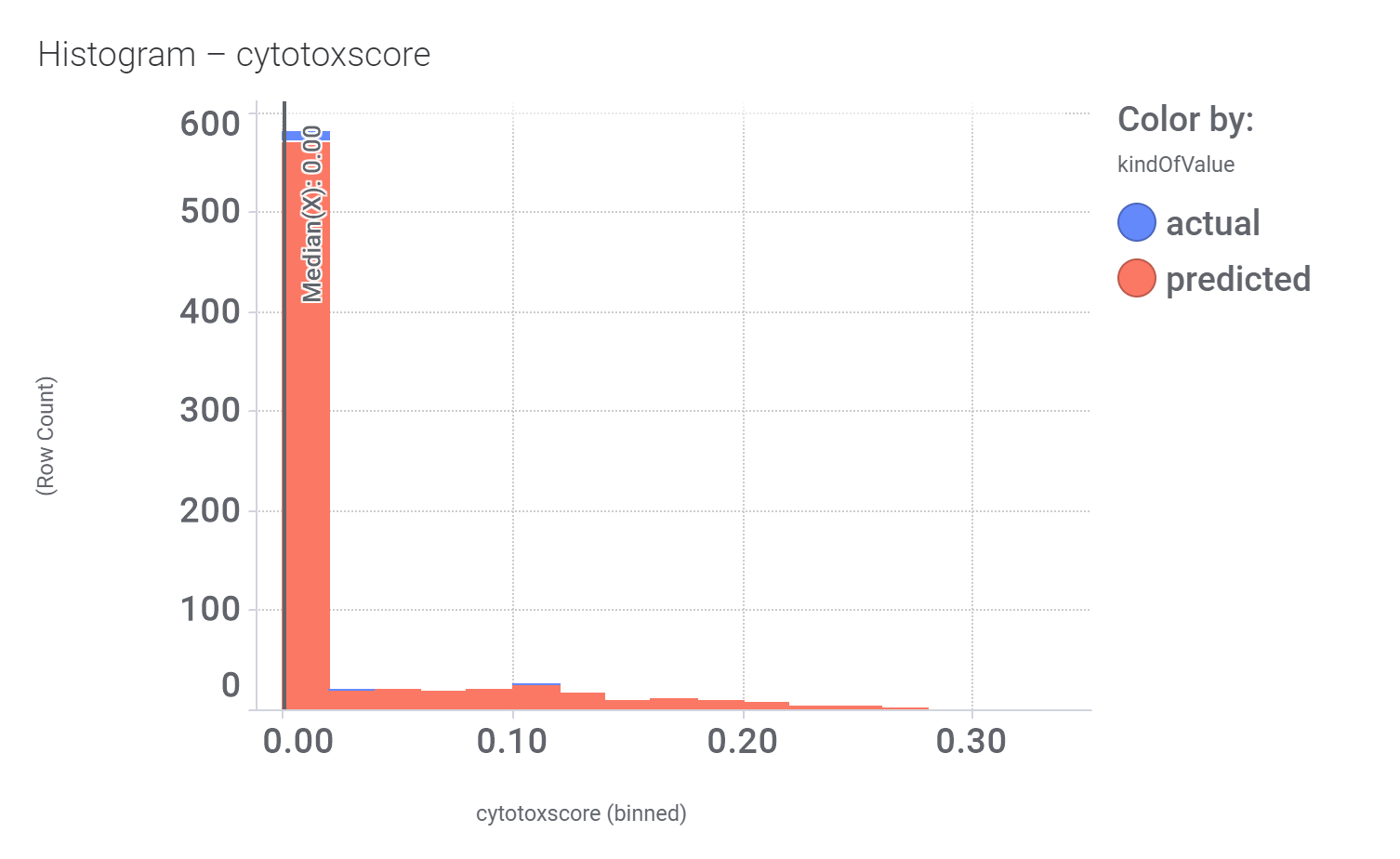
Among the predicted GLP1R PAM actives there are > 700 compounds with > 70% similarity with LY3502970. Some exemplars here.
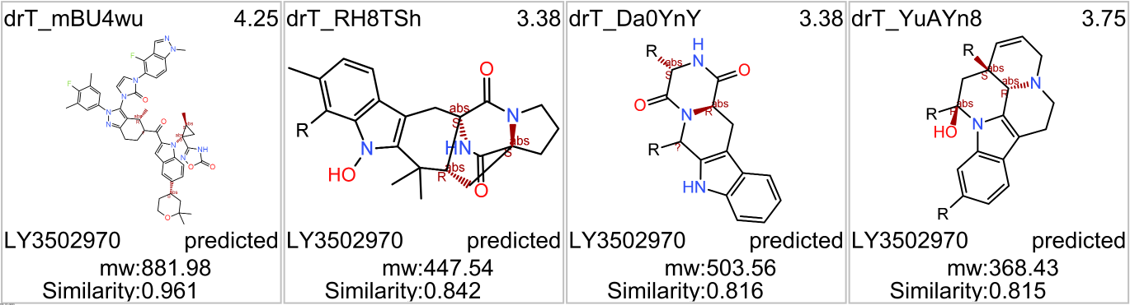
We can examine the effect of these molecules on relevant ChEMBL phenotypes using interaction graphs…
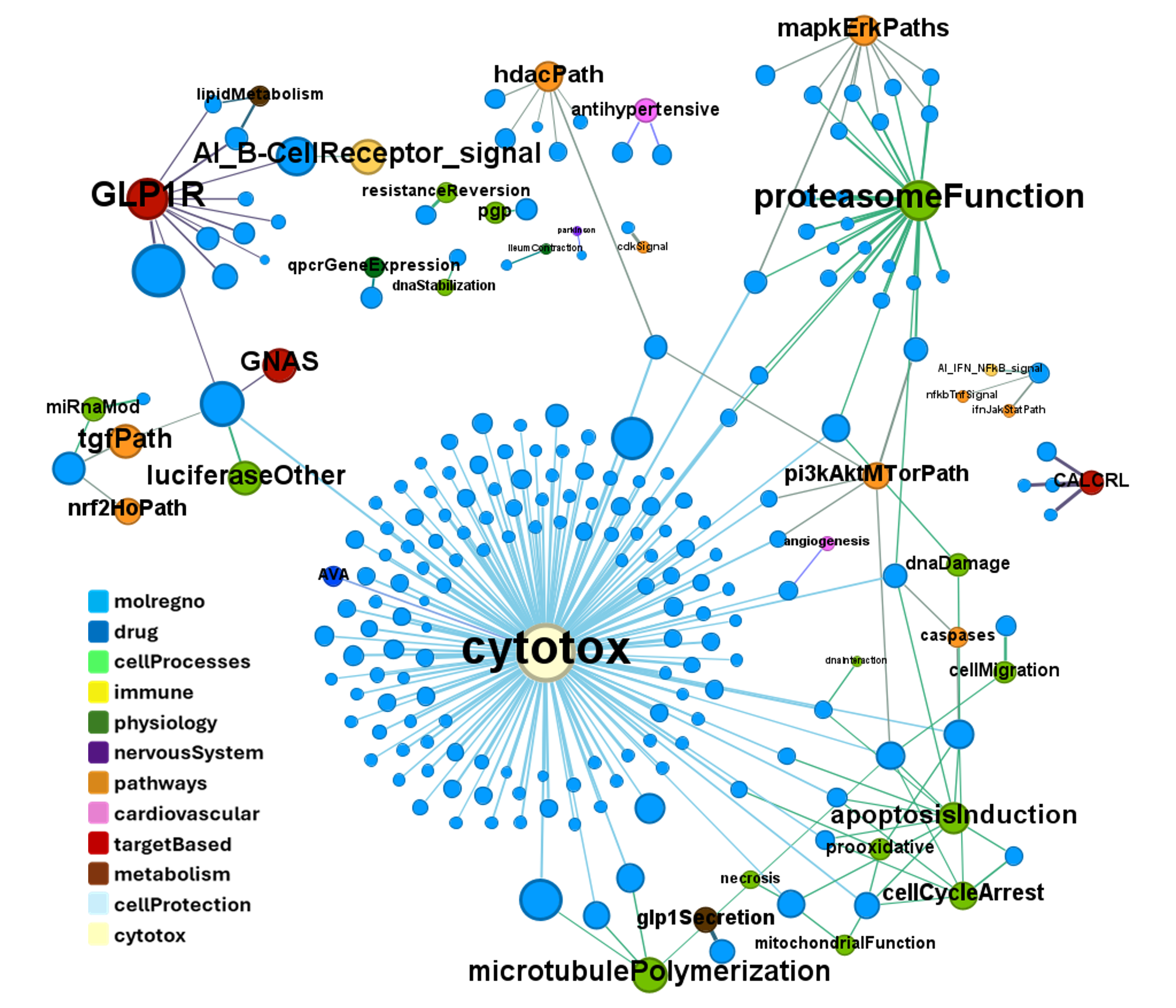
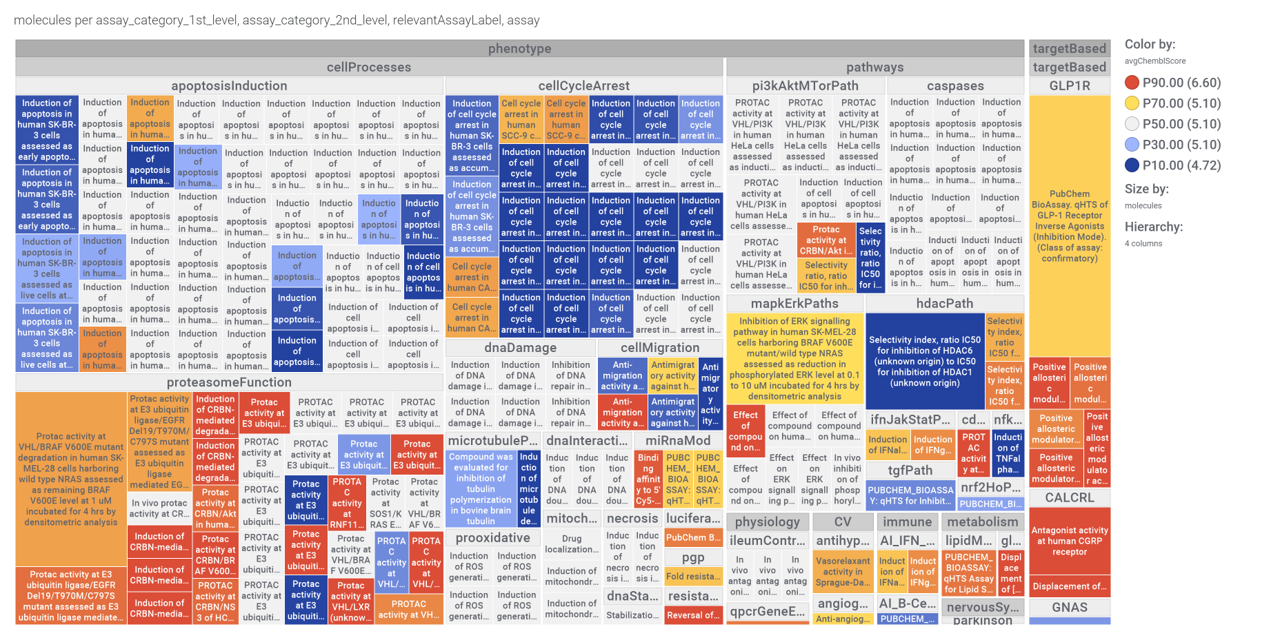
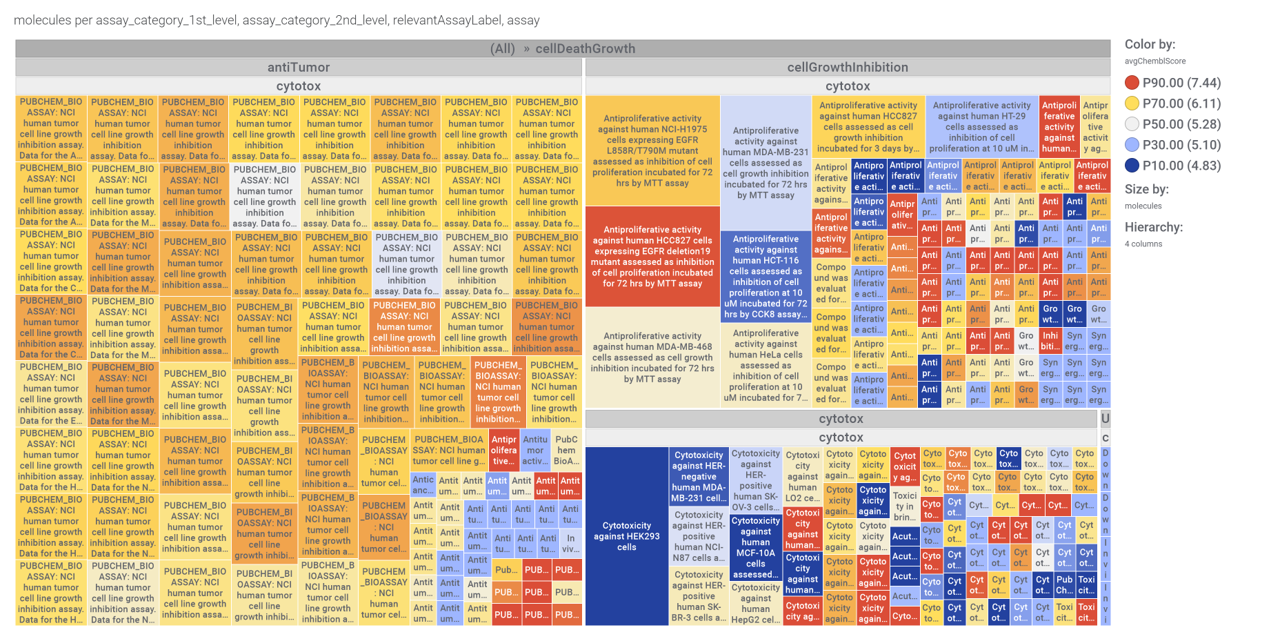
…and inspect specific assays involved in such phenotypes with treeMaps.
For target-based assays and phenotypes…
… or cytotoxicity.
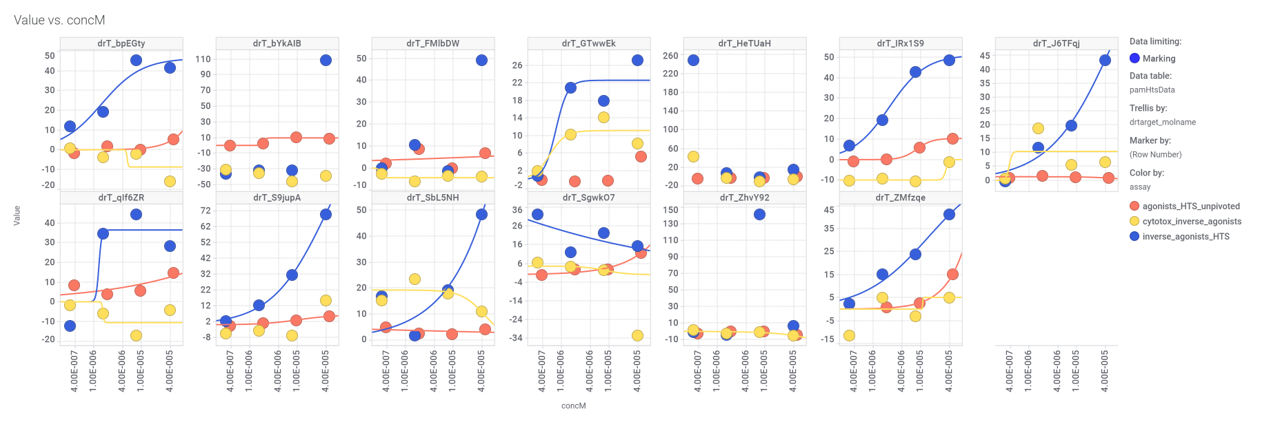
Some of these compounds have also full curve data from PubChem GLP1R screens.
And finally, we can have a look on some of their bio-physicochemical and developability properties.
Druglikeness estimated by QedWeighted.
Druglikeness estimated by DataWarrior.
Some references included
Br J Pharmacol. 2022 Feb; 179(4): 511–525. doi: 10.1111/bph.15446
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
2.4. LY3502970 (OWL‐833) and CHU‐128
In search of low MW activators of GLP‐1 receptors, Kawai et al. at Chuai Pharmaceutical and Eli Lilly and Company used a screening method that detects compound‐induced expression of a urokinase‐type plasminogen activator in LLC‐PK1 cells expressing human GLP‐1 receptors (Tamura et al., 2016), followed by multiple rounds of SAR work to optimize affinity and drug‐like properties. These led to the discovery of compound 4 (LY3502970, also known as OWL‐833) (Figure 1, [4]) (Yoshino et al., 2018). In vitro studies using HEK293 cells expressing various densities of human GLP‐1 receptors reveals that LY3502970 is a partial agonist of these receptors, biased towards cAMP accumulation with no detectable β‐arrestin recruitment. It is much more potent than TT‐OAD2 in stimulating GLP‐1 receptor‐mediated cAMP accumulation and is highly selective against other Class B GPCRs. In vivo studies show that oral administration of LY3502970 results in glucose lowering in humanized GLP‐1 receptor transgenic mice (use of these mice is essential due to the species specificity of the molecule; X. Zhang et al., 2020) and insulinotropic and hypophagic effects in non‐human primates. Given its favourable pharmacokinetic profile and efficacy, LY3502970 is currently being evaluated in early stage clinical trial for its potential as an antidiabetic agent (ClinicalTrials.gov Identifier: NCT04426474). From the same patent series by Chugai, compound 5 (CHU‐128) (Figure 1, [5]) has also been studied in vitro (X. Zhang et al., 2020). Given that the chemical structure of CHU‐128 is very close to that of LY3502970, it is not surprising to find that CHU‐128 has a pharmacological profile similar to that of LY3502970.
In the recently reported cryo‐EM structure of GLP‐1 receptors with LY3502970, GsiN18, antibody Nb35 and single‐chain variable fragment scFv16, the compound LY3502970 binds in the helical bundle that partlly overlaps with the area where TT‐OAD2 binds and interacts with residues within the ECD, TM1–TM3, ECL2 and TM7 (Figure 2) (Kawai et al., 2020). The binding of LY3502970 results in a distinct conformation of the ECD and the extracellular portion of the 7TM segments. The overall binding modes between LY3502970 and TT_OAD2 are different. TT_OAD2 adopts a U‐shaped orientation with both ends of its backbone sitting between TM2, TM3 and ECL1 (Zhao et al., 2020). For LY3502970, its 2,2‐dimethyl‐tetrahydropyran moiety occupies a similar position as the 2,3‐dime‐thylpyridine ring of TT‐OAD2, whereas its 4‐fluoro‐1‐methyl‐indazole moiety at the other end extends between TM1 and TM2. Further, its 3,5‐dimethyl‐4‐fluoro‐phenyl ring interacts with TM1 and TM7. Similarly, compound CHU‐128 adopts the same binding mode as LY3502970.
Nat Commun. 2016; 7: 13384. doi: 10.1038/ncomms13384
Identification of an orally active small-molecule PTHR1 agonist for the treatment of hypoparathyroidism
Tatsuya Tamura,a,1 Hiroshi Noda,1 Eri Joyashiki,1 Maiko Hoshino,1 Tomoyuki Watanabe,1 Masahiko Kinosaki,1 Yoshikazu Nishimura,1 Tohru Esaki,1 Kotaro Ogawa,1 Taiji Miyake,1 Shinichi Arai,1 Masaru Shimizu,1 Hidetomo Kitamura,1 Haruhiko Sato,1 and Yoshiki Kawabe1
Abstract
Parathyroid hormone (PTH) is essential for calcium homeostasis and its action is mediated by the PTH type 1 receptor (PTHR1), a class B G-protein-coupled receptor. Hypoparathyroidism and osteoporosis can be treated with PTH injections; however, no orally effective PTH analogue is available. Here we show that PCO371 is a novel, orally active small molecule that acts as a full agonist of PTHR1. PCO371 does not affect the PTH type 2 receptor (PTHR2), and analysis using PTHR1–PTHR2 chimeric receptors indicated that Proline 415 of PTHR1 is critical for PCO371-mediated PTHR1 activation. Oral administration of PCO371 to osteopenic rats provokes a significant increase in bone turnover with limited increase in bone mass. In hypocalcemic rats, PCO371 restores serum calcium levels without increasing urinary calcium, and with stronger and longer-lasting effects than PTH injections. These results strongly suggest that PCO371 can provide a new treatment option for PTH-related disorders, including hypoparathyroidism.
Selectivity assay
Selectivity assays against class B GPCRs were performed by Cerep (France). Agonistic and antagonistic effects of PCO371 against a panel of 12 class B GPCRs (CGRP, calcitonin, CRF1, CRF2α, GLP-1, GLP-2, glucagon, secretin, GHRH, PAC1, VPAC1 and VPAC2) were examined at PCO371 concentrations of 1 and 10 μmol l−1. The following cell lines were used; T47D (calcitonin), βTC6 (GLP-1), HT-29 (VPAC1, VPAC2), CHO (CGRP, CRF1, GLP-2, Glucagon, Secretin, GHRH and PAC1, recombinant), and HEK-293 (CRF-2, recombinant).
j.molcel.2020.09.020. Epub 2020 Oct 6. Doi: 10.1016/j.molcel.2020.09.020
Differential GLP-1R Binding and Activation by Peptide and Non-peptide Agonists
Xin Zhang 1, Matthew J Belousoff 1, Peishen Zhao 1, Albert J Kooistra 2, Tin T Truong 1, Sheng Yu Ang 1, Christina Rye Underwood 3, Thomas Egebjerg 3, Petr Šenel 4, Gregory D Stewart 1, Yi-Lynn Liang 1, Alisa Glukhova 1, Hari Venugopal 5, Arthur Christopoulos 1, Sebastian G B Furness 1, Laurence J Miller 6, Steffen Reedtz-Runge 3, Christopher J Langmead 1, David E Gloriam 2, Radostin Danev 7, Patrick M Sexton 8, Denise Wootten 9
Abstract
Peptide drugs targeting class B1 G-protein-coupled receptors (GPCRs) can treat multiple diseases; however, there remains substantial interest in the development of orally delivered non-peptide drugs. Here, we reveal unexpected overlap between signaling and regulation of the glucagon-like peptide-1 (GLP-1) receptor by the non-peptide agonist PF 06882961 and GLP-1 that was not observed for another compound, CHU-128. Compounds from these patent series, including PF 06882961, are currently in clinical trials for treatment of type 2 diabetes. High-resolution cryoelectron microscopy (cryo-EM) structures reveal that the binding sites for PF 06882961 and GLP-1 substantially overlap, whereas CHU-128 adopts a unique binding mode with a more open receptor conformation at the extracellular face. Structural differences involving extensive water-mediated hydrogen bond networks could be correlated to functional data to understand how PF 06882961, but not CHU-128, can closely mimic the pharmacological properties of GLP-1. These findings will facilitate rational structure-based discovery of non-peptide agonists targeting class B GPCRs.