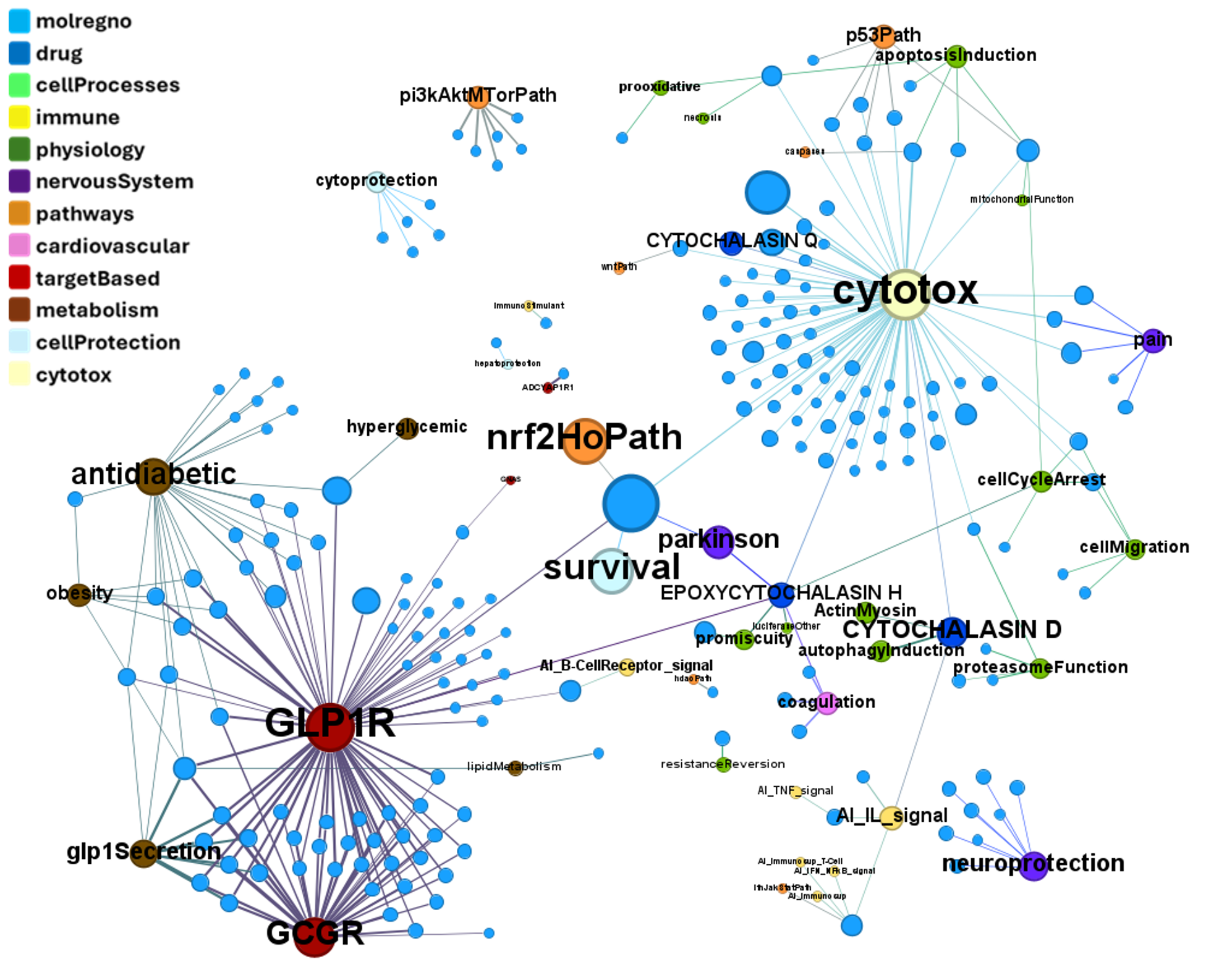
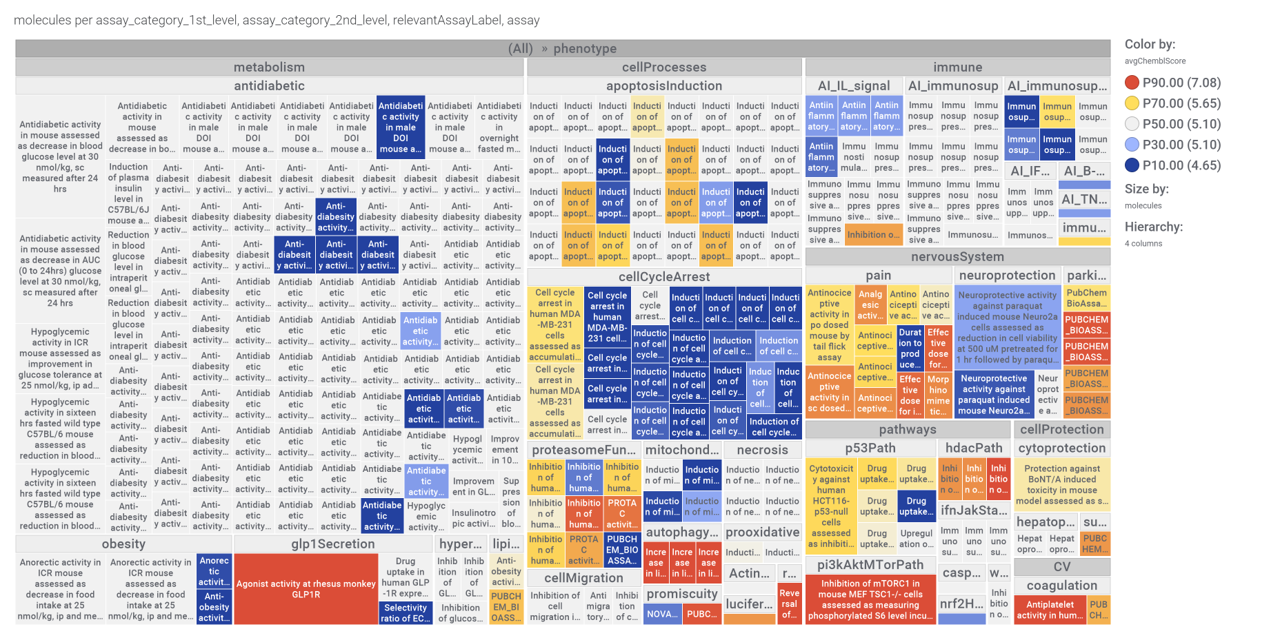
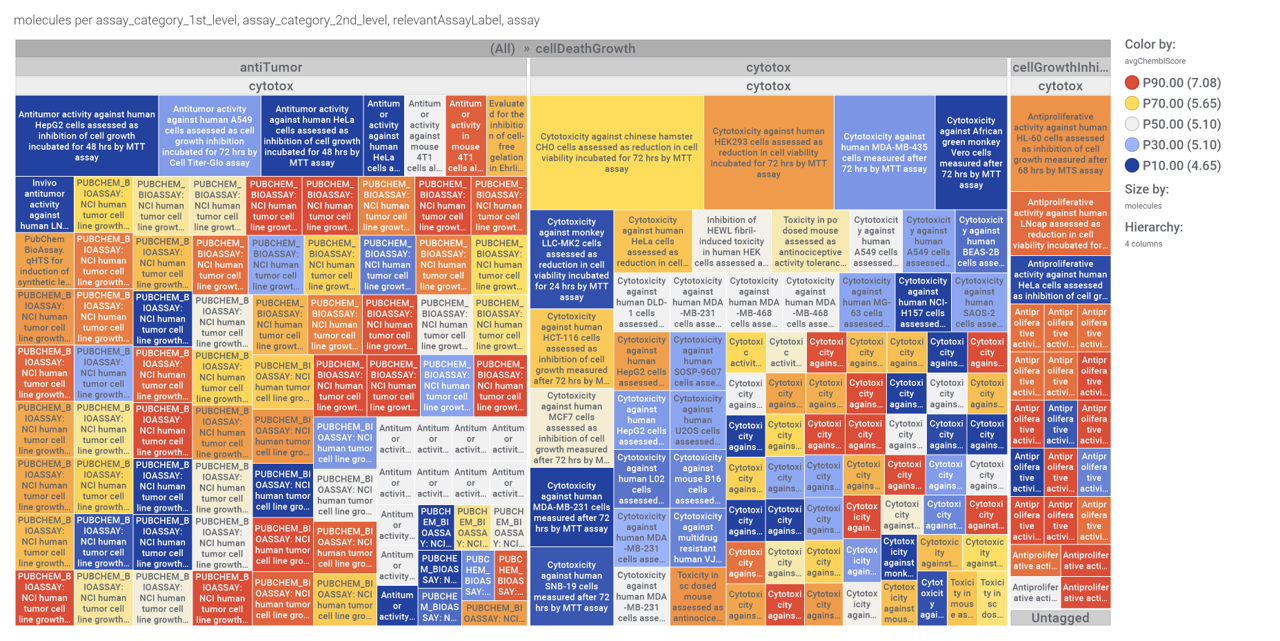
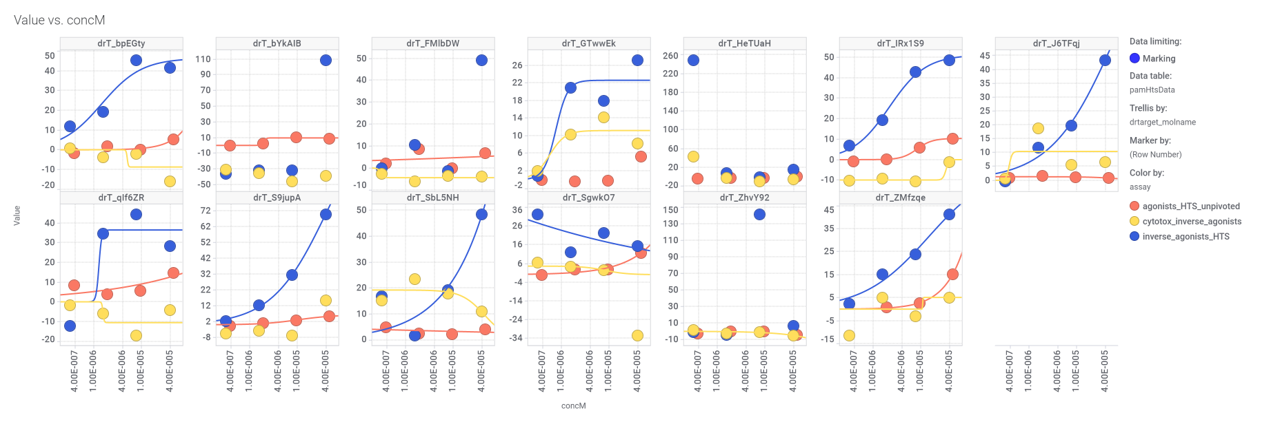
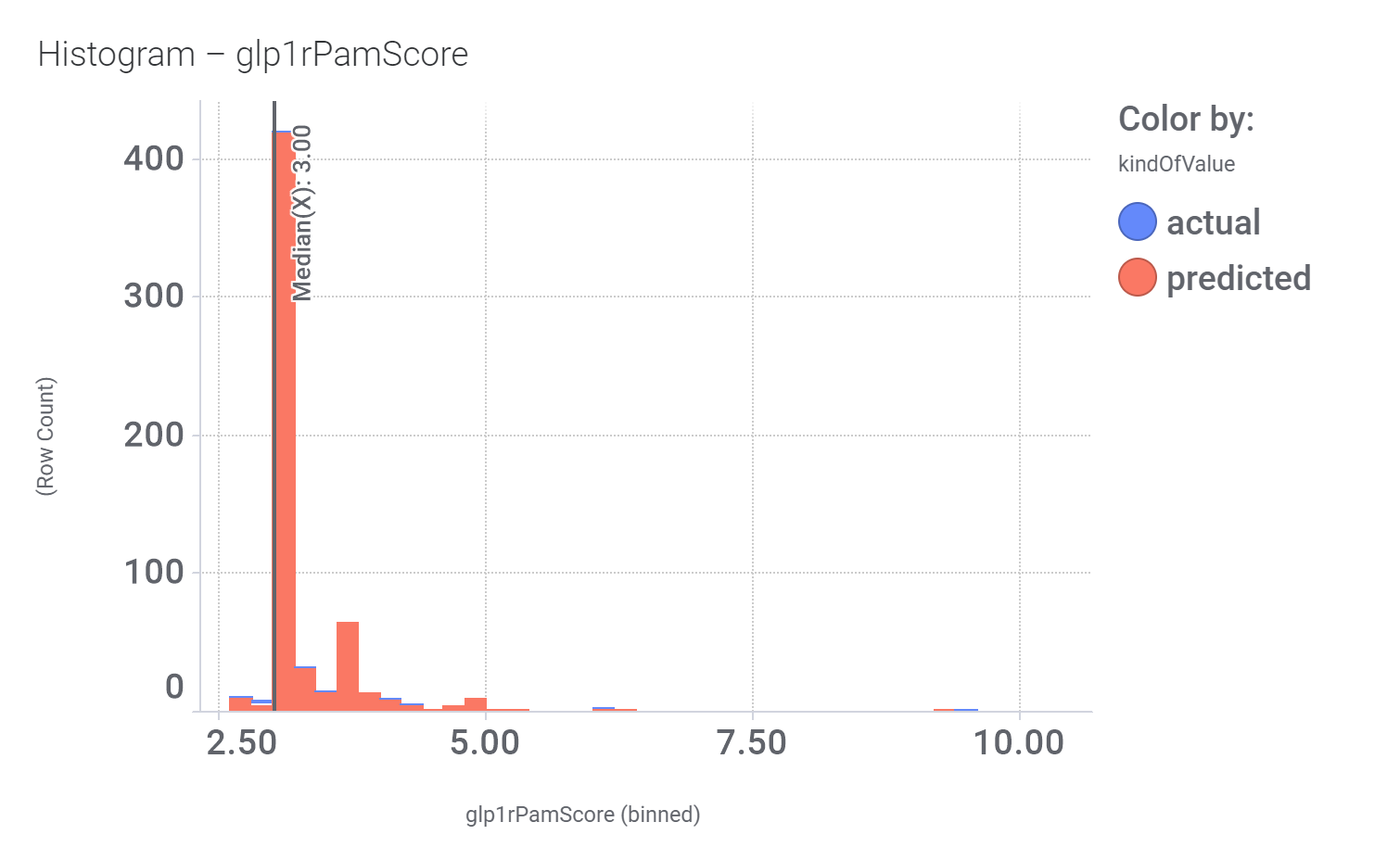
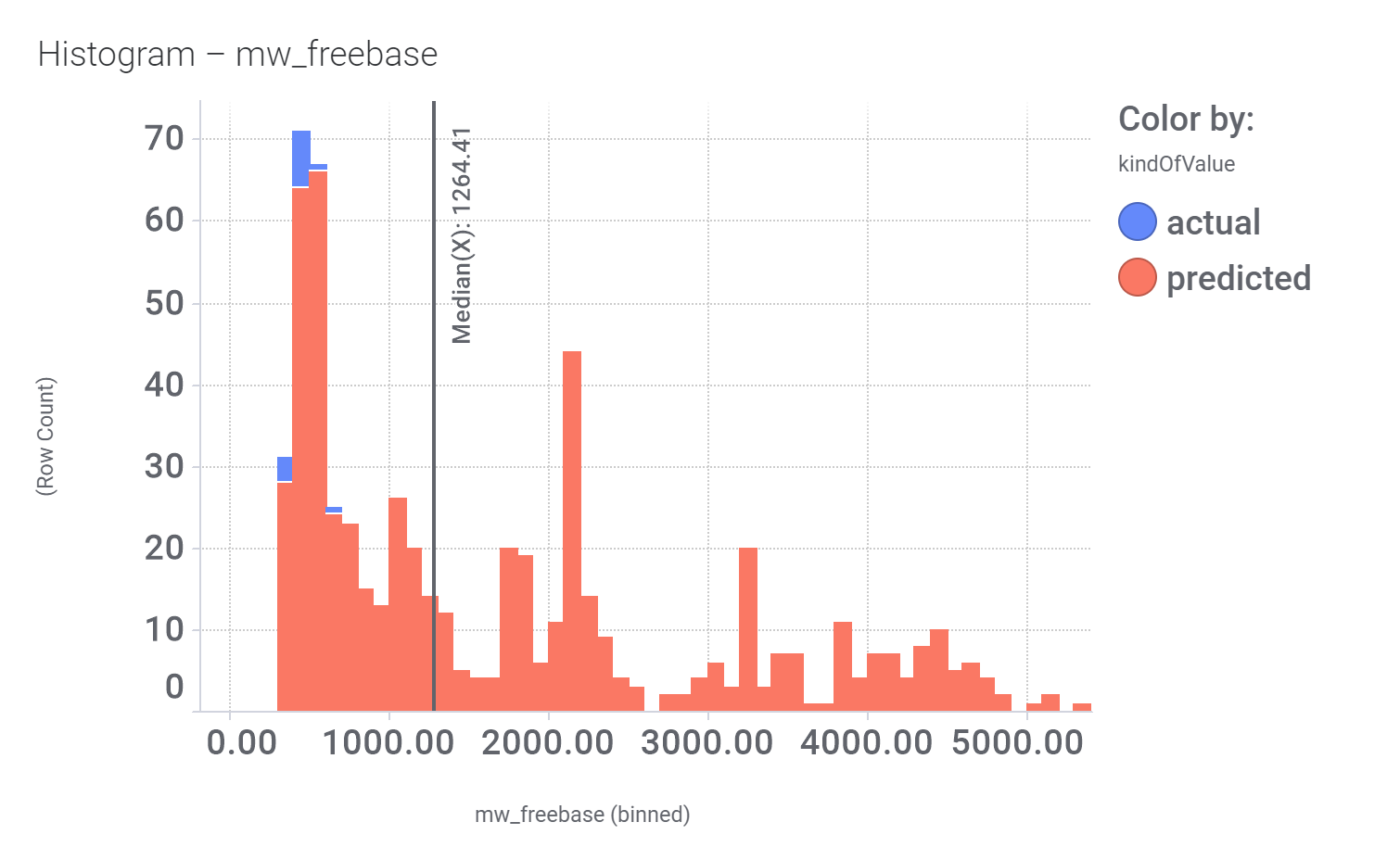
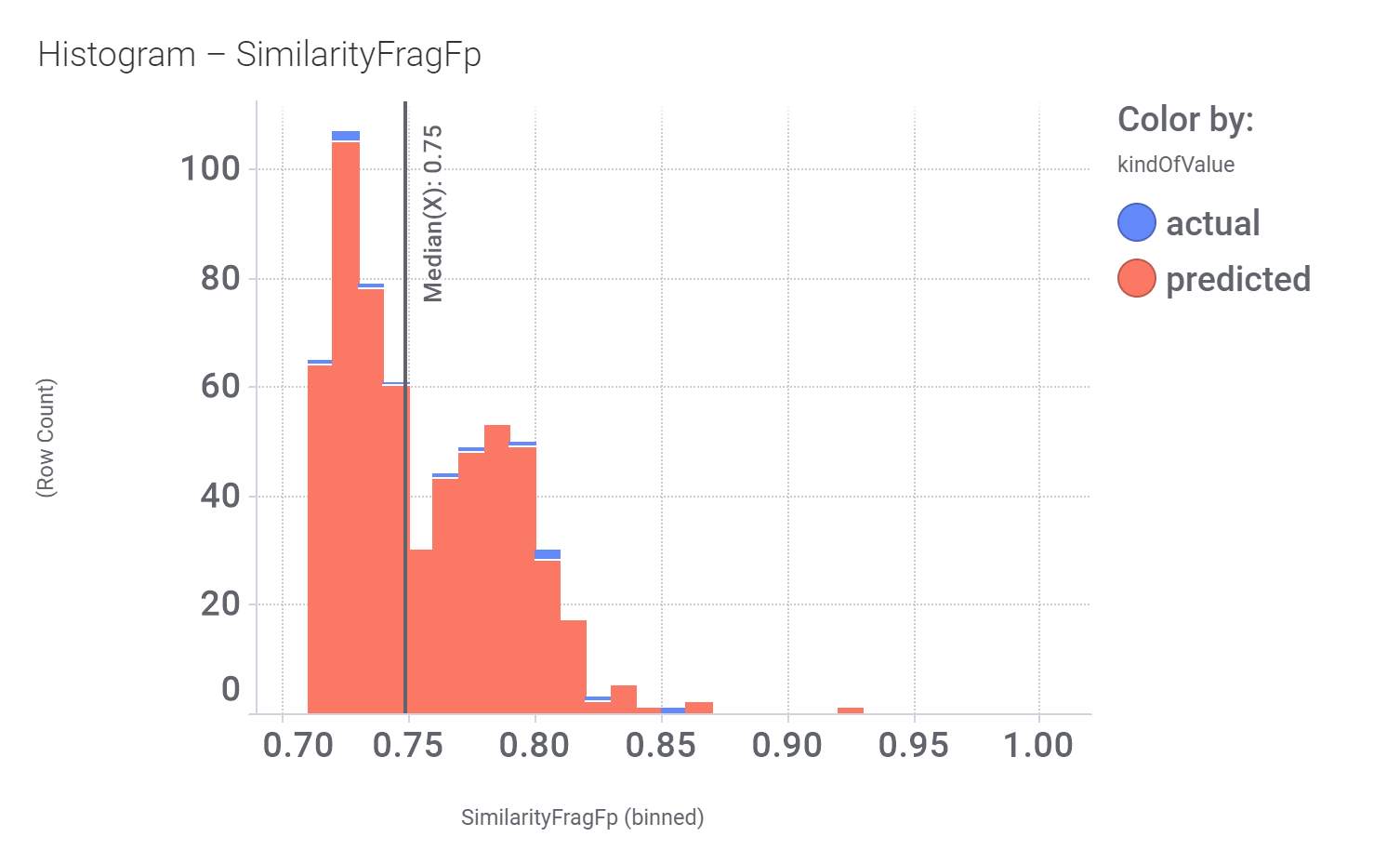
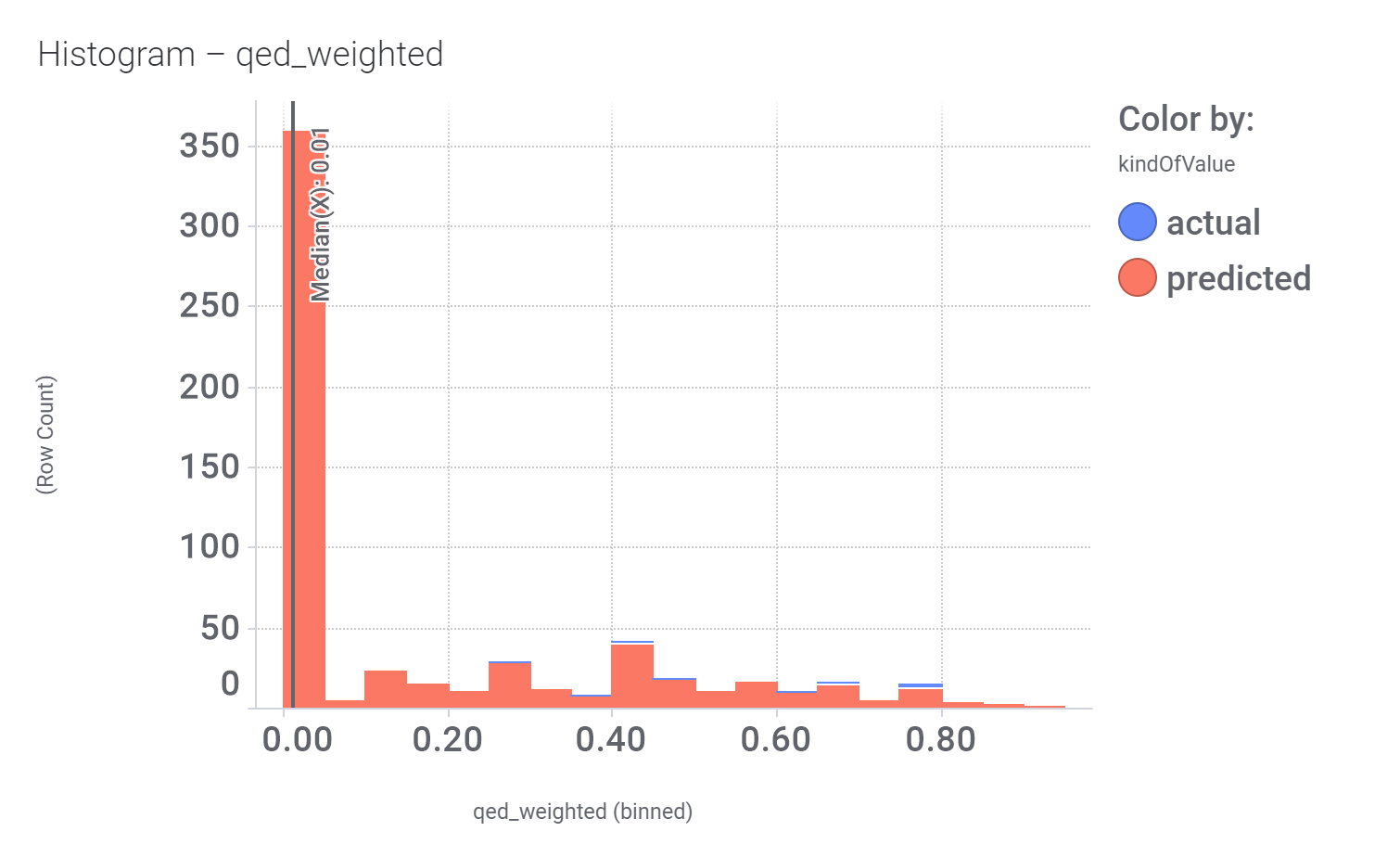
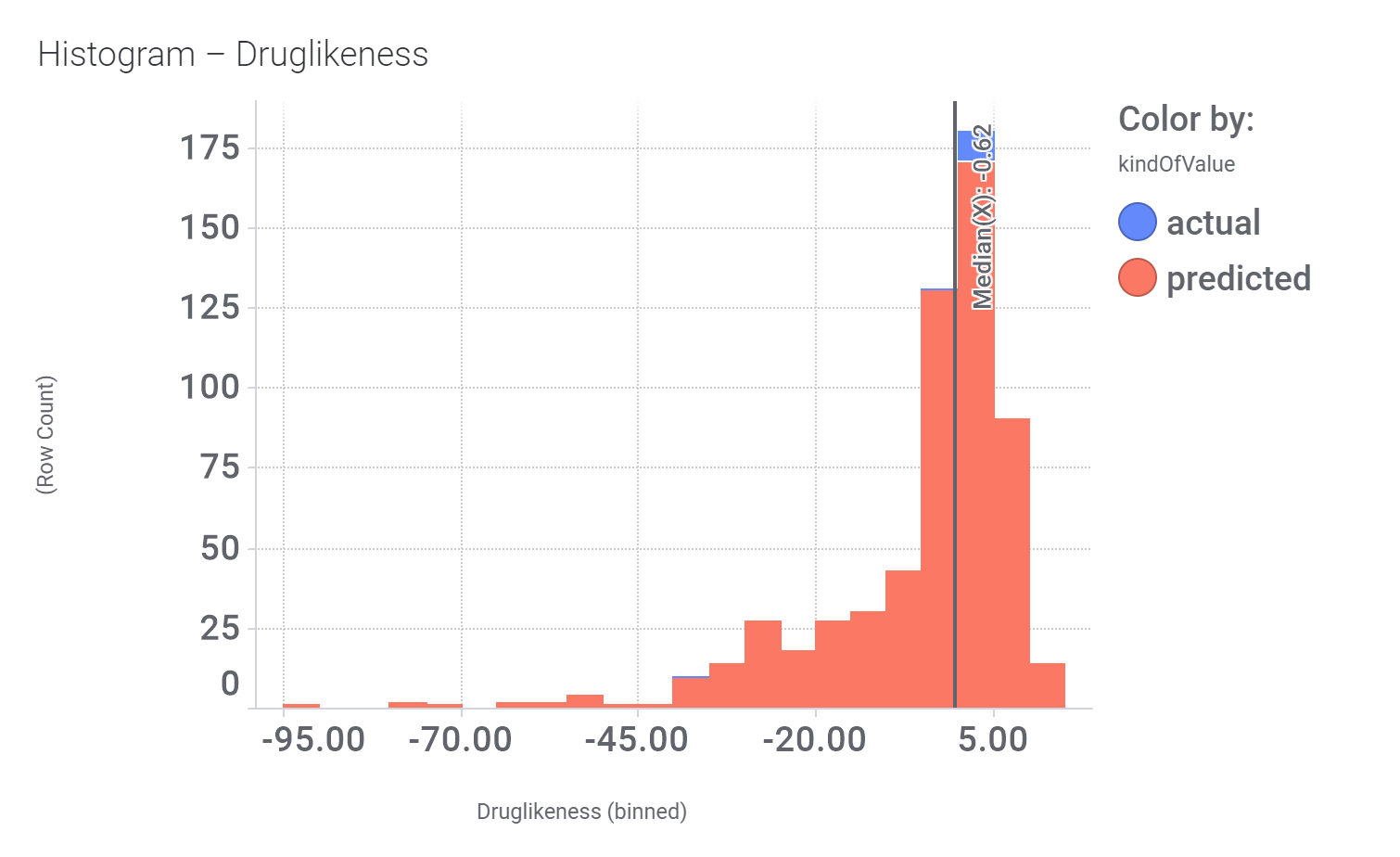
We can examine the effect of these molecules on relevant ChEMBL phenotypes using interaction graphs…
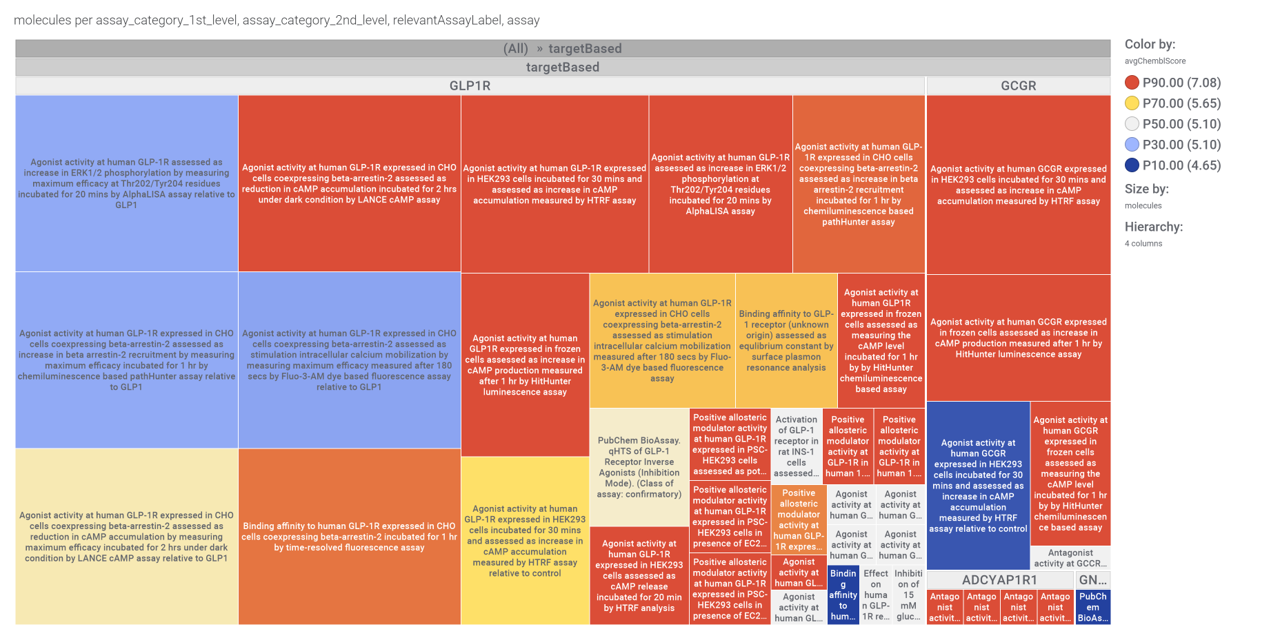
…and inspect specific assays involved in such phenotypes with treeMaps.
For relevant target based assays
ChEMBL phenotypes
Or cytotoxicity assays
Some of these compounds have also full curve data from PubChem GLP1R screens.
And finally, we can have a look on some of their bio-physicochemical and developability properties.
Druglikeness estimated by QedWeighted.
Druglikeness estimated by DataWarrior.
Some references included
Br J Pharmacol. 2022 Feb; 179(4): 511–525. Published online 2021 Apr 19. doi: 10.1111/bph.15446. PMID: 33724441
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
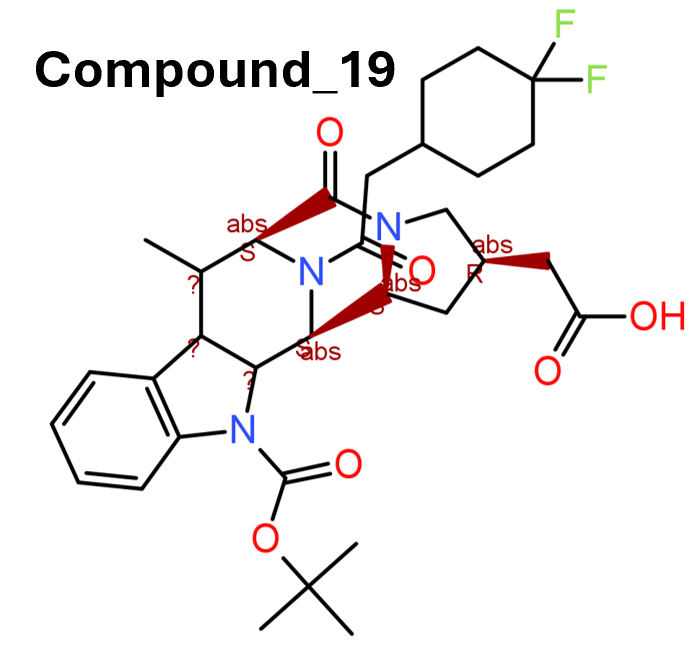
3.1.8. Compound 19
In another effort to take advantage of the increased bioavailability and longer half‐life of GLP‐1(9–36)NH2, a group at Sanofi‐Aventis Deutschland GmbH performed HT screening of its own compound collection using an HTRF cAMP assay in a HEK293 cell line overexpressing the human GLP‐1 receptor, which was followed by chemical optimization of the lead compounds (Méndez et al., 2020). These efforts led to the discovery of compound 14 (compound 19) (Figure 3, [14]) based on a 3,4,5,6‐tetrahydro‐1H‐1,5‐epiminoazocino[4,5‐b]indole scaffold. In vitro assay demonstrated that compound 19 is a potent GLP‐1 receptor PAM by clearly potentiating GLP(9–36)NH2 with EC50 value of 5 nM. In the endogenous pancreatic beta‐cell line 1.1B4 cells, compound 19 also shows robust PAM efficacy. In dispersed rat pancreatic islets, compound 19 significantly improved the GLP‐1(9–36)NH2‐mediated glucose‐stimulated insulin secretion. In vivo pharmacokinetic and pharmacological characterization indicated that compound 19 allosterically activated GLP‐1 receptors in the presence of GLP(9–36)NH2. To date, compound 19 is the most potent non‐covalent PAM of GLP‐1 receptors reported, although the exogenous addition of GLP(9–36)NH2 is still needed in order to elicit a significant response on glucose homeostasis in vivo.
. 2020 Mar 12;63(5):2292-2307. doi: 10.1021/acs.jmedchem.9b01071.
Design, Synthesis, and Pharmacological Evaluation of Potent Positive Allosteric Modulators of the Glucagon-like Peptide-1 Receptor (GLP-1R)
Affiliation
- 1Sanofi-Aventis Deutschland GmbH, Industriepark Höchst, 65926 Frankfurt, Germany.
Abstract
The therapeutic success of peptidic GLP-1 receptor agonists for treatment of type 2 diabetes mellitus (T2DM) motivated our search for orally bioavailable small molecules that can activate the GLP-1 receptor (GLP-1R) as a well-validated target for T2DM. Here, the discovery and characterization of a potent and selective positive allosteric modulator (PAM) for GLP-1R based on a 3,4,5,6-tetrahydro-1H-1,5-epiminoazocino[4,5-b]indole scaffold is reported. Optimization of this series from HTS was supported by a GLP-1R ligand binding model. Biological in vitro testing revealed favorable ADME and pharmacological profiles for the best compound 19. Characterization by in vivo pharmacokinetic and pharmacological studies demonstrated that 19 activates GLP-1R as positive allosteric modulator (PAM) in the presence of the much less active endogenous degradation product GLP1(9-36)NH2 of the potent endogenous ligand GLP-1(7-36)NH2. While these data suggest the potential of small molecule GLP-1R PAMs for T2DM treatment, further optimization is still required towards a clinical candidate.