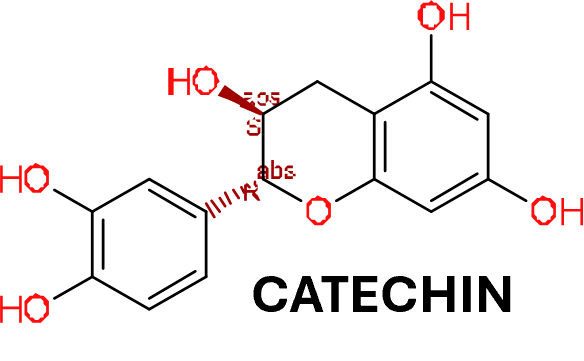
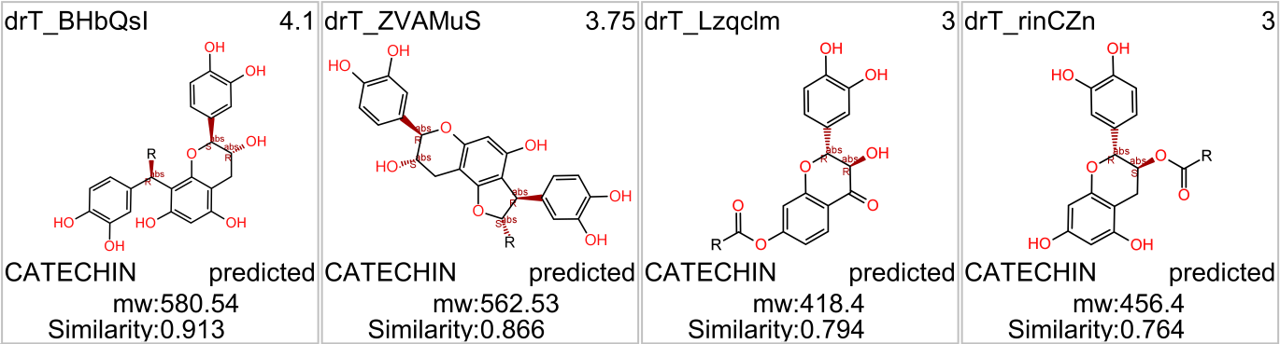
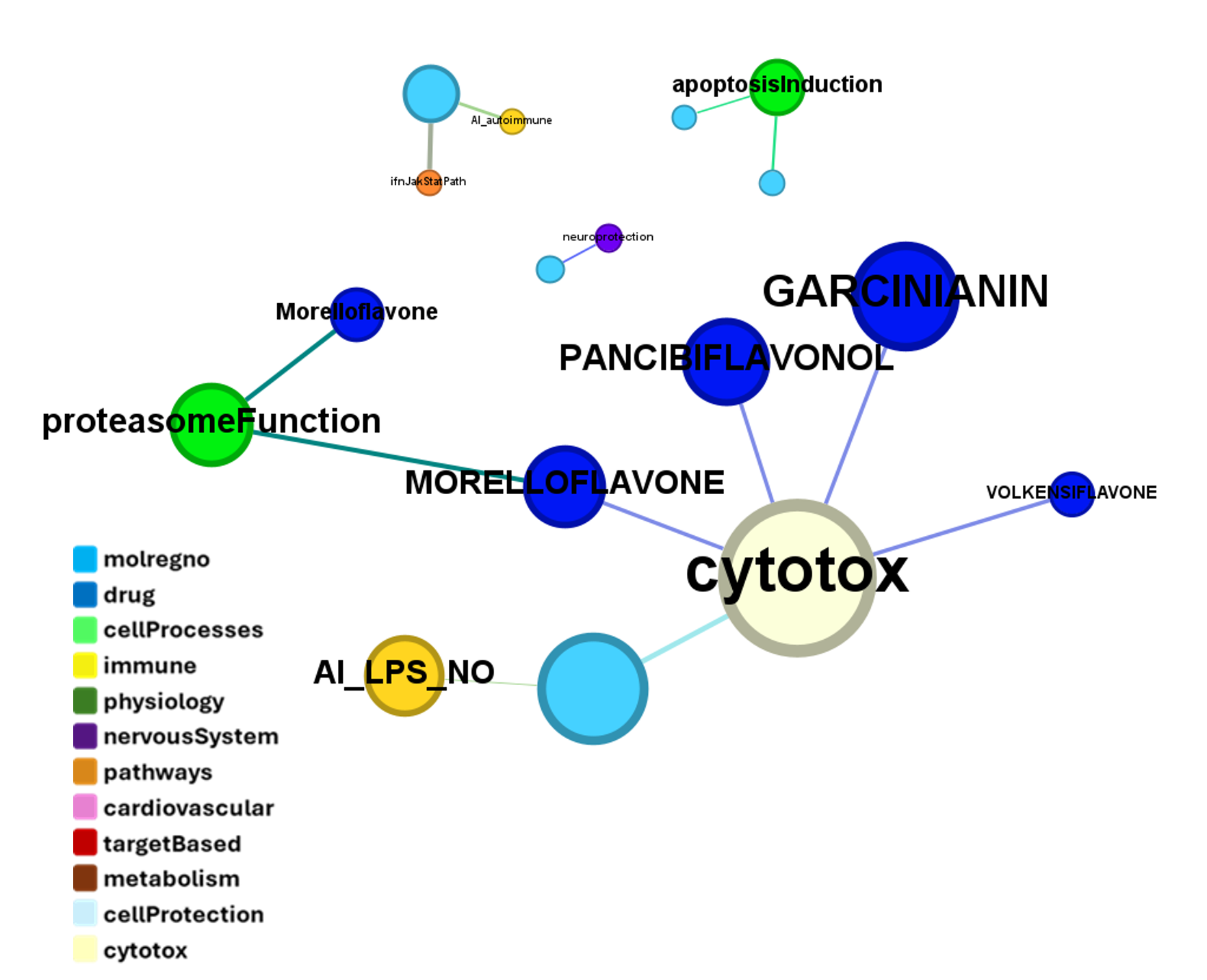
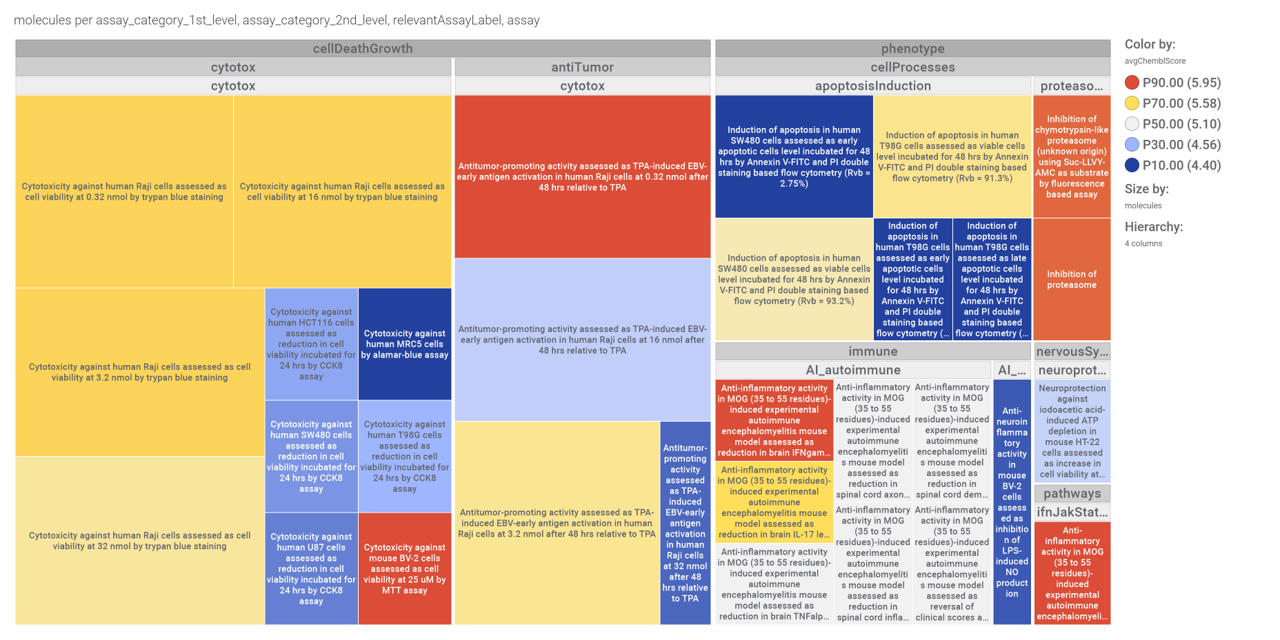
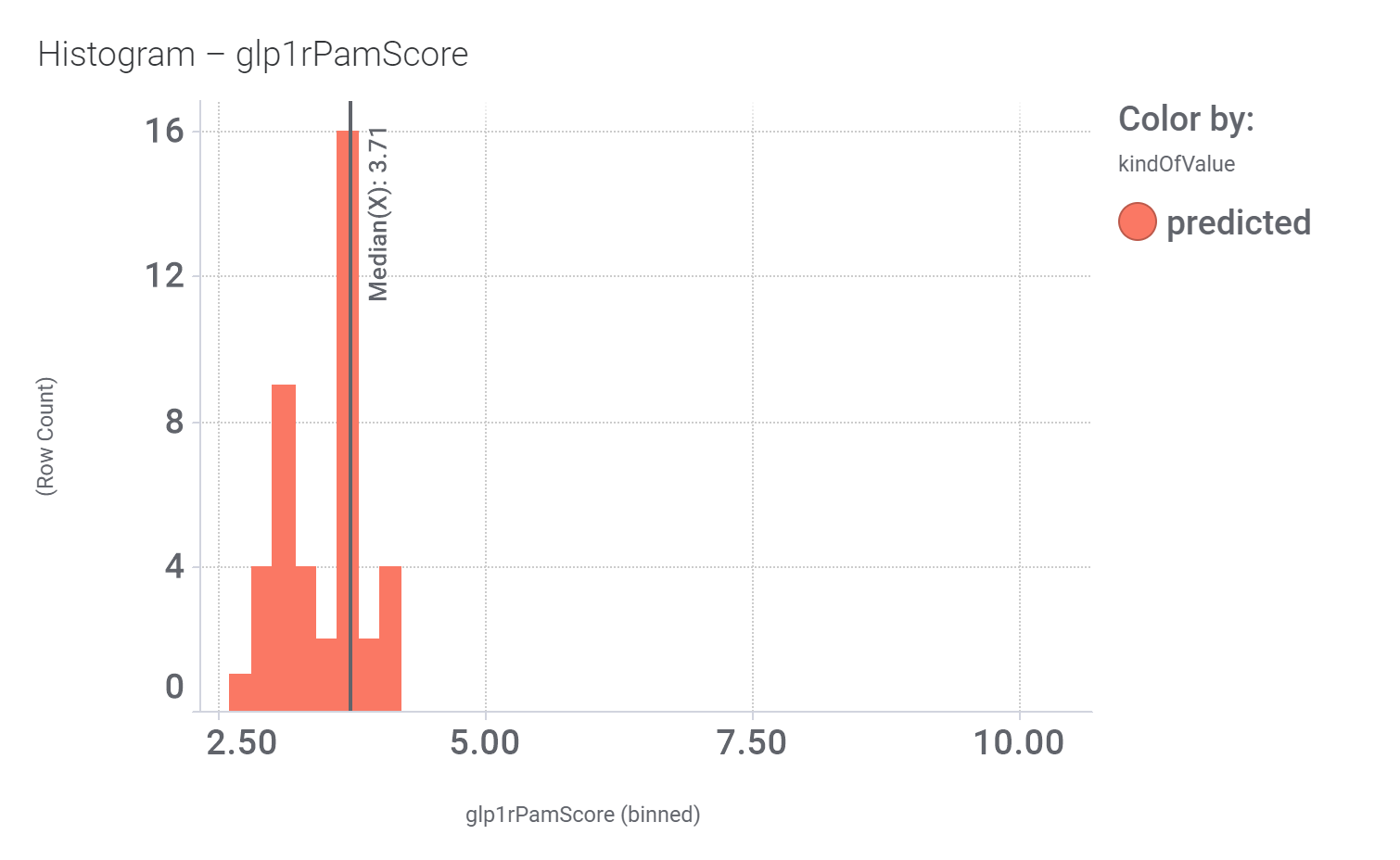
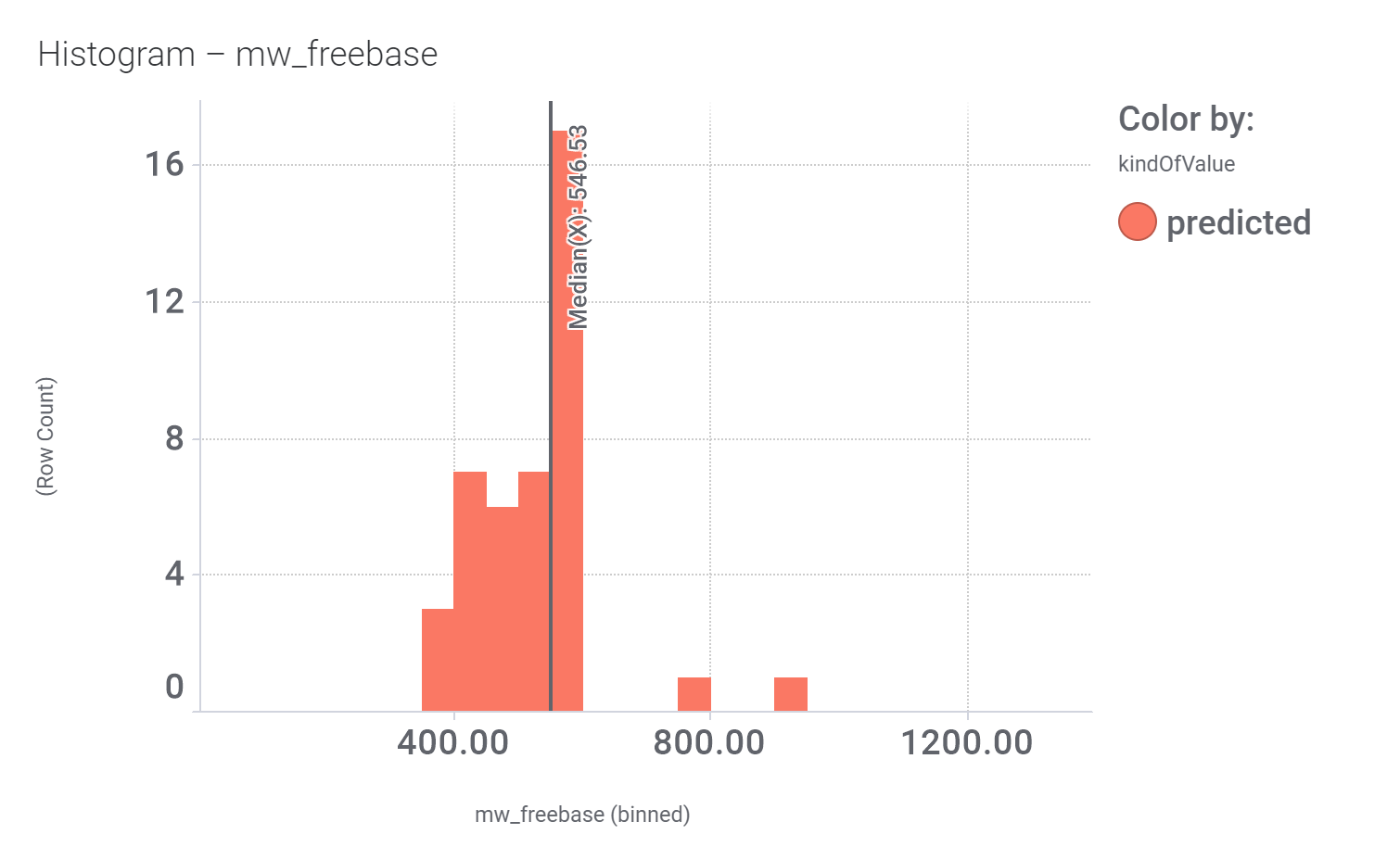
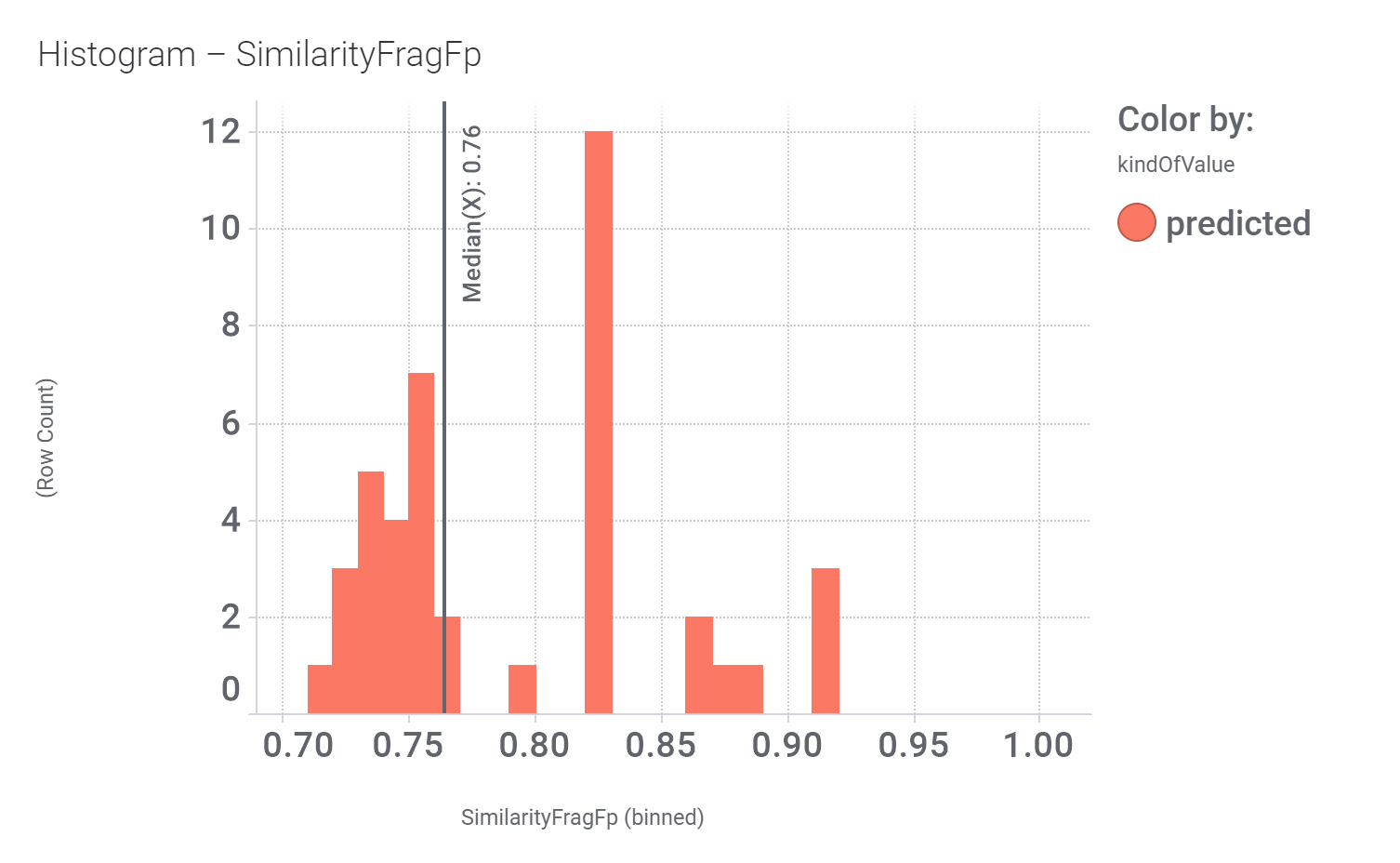
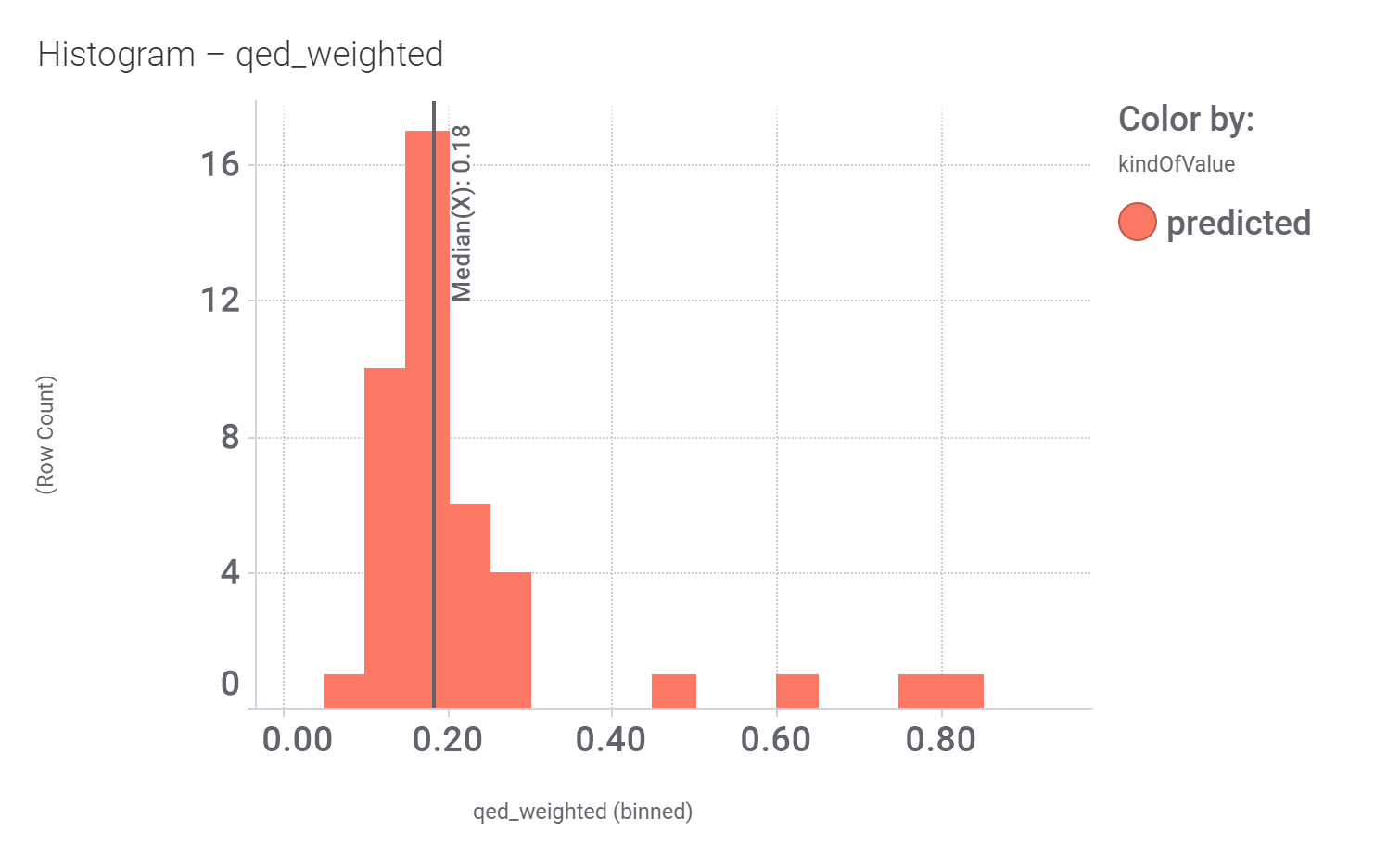
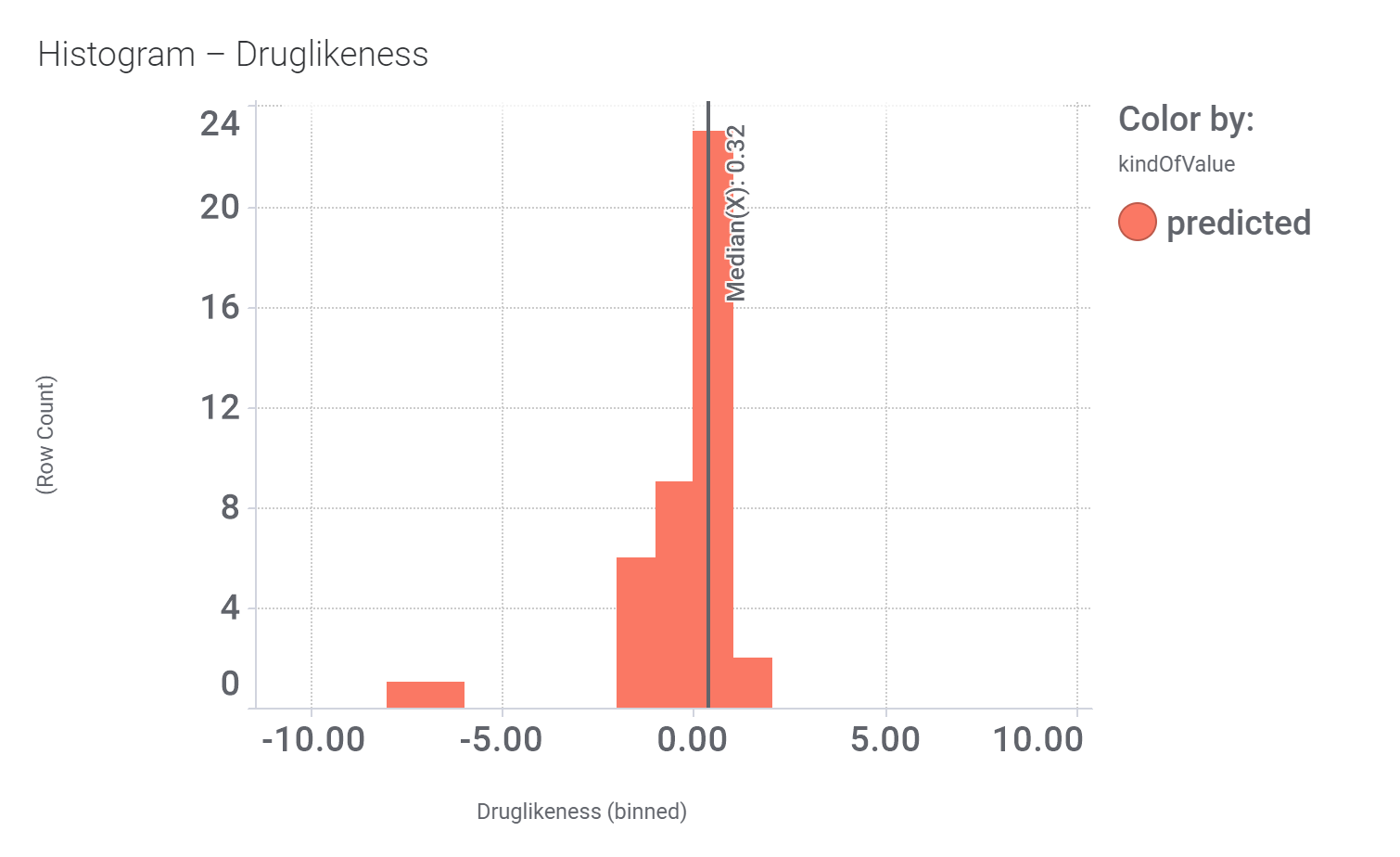
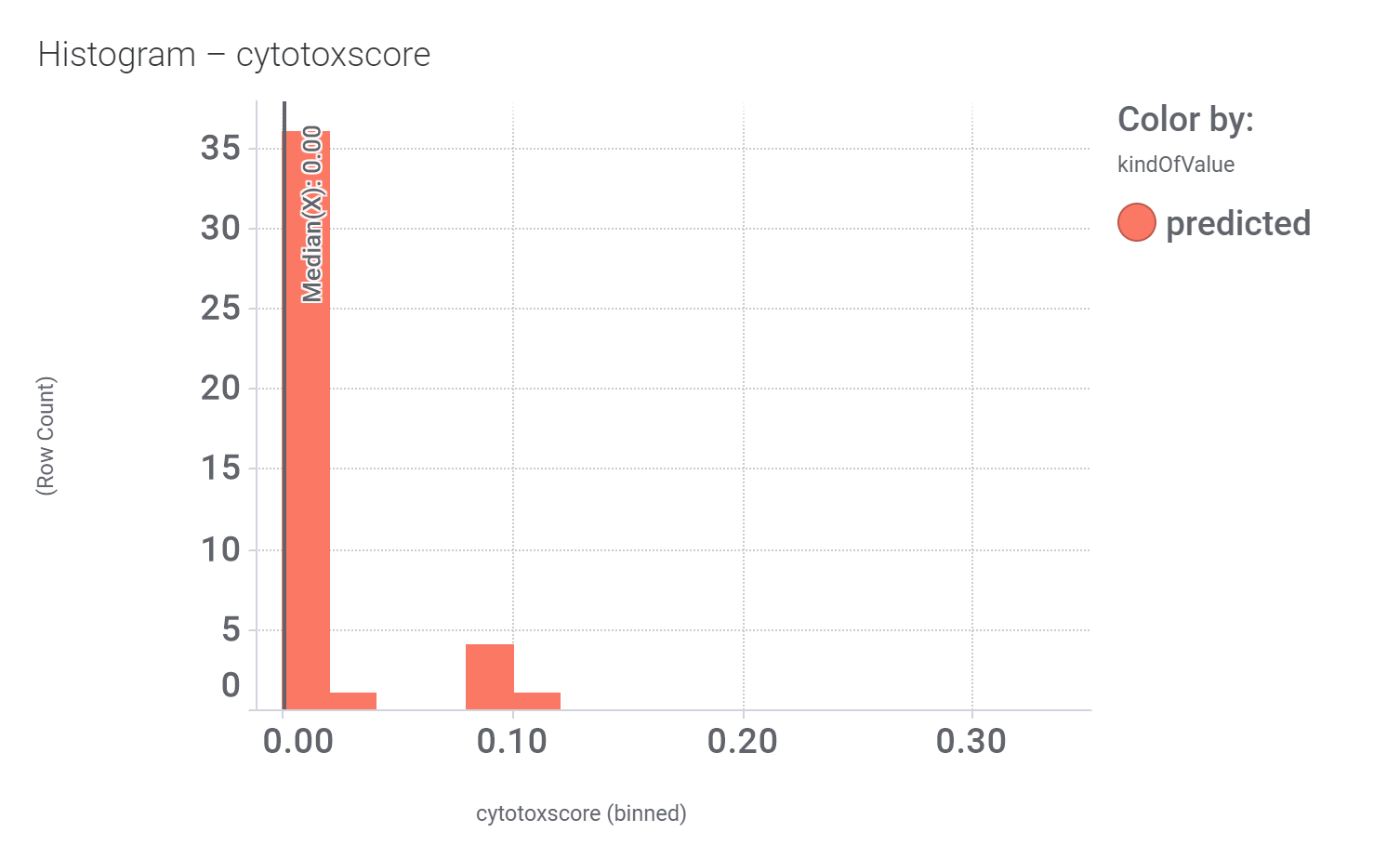
in 2009, Domain Therapeutics reported a series of quercetin‐like flavonoids as PAMs of GLP‐1 receptors from which a series of hydroxyl flavonols and catechin were further characterized as GLP1R PAMs. DrTarget has predicted GLP1RPAM activity for 42 catechin analogs.
See exemplars here.
These compounds show few interactions with ChEMBL phenotypes.
In a limited number of assays
And these are their bio-physicochemical and developability properties.
Literature references
Br J Pharmacol. 2022 Feb; 179(4): 511–525. Published online 2021 Apr 19. doi: 10.1111/bph.15446. PMID: 33724441
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Author information Article notes Copyright and License information PMC Disclaimer
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
3.1.2. Flavonoids
In search for GPCR allosteric modulators, Domain Therapeutics reported a series of quercetin‐like flavonoids as PAMs of GLP‐1 receptors (Schann et al., 2009). These compounds and other flavonoids such as flavones, isoflavones and catechin (compound 9) (Figure 3, [9]) were subsequently characterized by Koole et al., (2010) and Wootten et al. (2011). A series of hydroxyl flavonols and catechin were found to be probe dependent and induce biased signalling. Hydroxyl flavonols do not have an effect on cAMP signalling but selectively modulate Ca2+ signalling in a peptide‐agonist dependent way. The Ca2+ modulation of quercetin was only observed with truncated GLP‐1 peptide or exendin‐4 and not on oxyntomodulin or full‐length peptides. On the other hand, catechin shows no allosteric effects on peptide‐mediated Ca2+ signalling. However, catechin negatively modulates cAMP formation in the presence of truncated GLP‐1 peptide, but not with full‐length GLP‐1, oxyntomodulin or exendin‐4.
Furthermore, the effects of the hydroxyl flavonols and catechin were driven primarily by their effects on orthosteric ligand efficacy. Although the undesirable characteristics of these polyphenolic compounds preclude them from further optimization for pharmacological purposes, the discovery of this series of compounds is further evidence for the allosterically modulation of GLP‐1 receptor function.
J Pharmacol Exp Ther. 2011 Feb;336(2):540-50. PMID: 21075839. DOI: 10.1124/jpet.110.176362
Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids
Denise Wootten 1, John Simms, Cassandra Koole, Owen L Woodman, Roger J Summers, Arthur Christopoulos, Patrick M Sexton
Abstract
The glucagon-like peptide 1 receptor (GLP-1R) is a promising target for the treatment of type II diabetes mellitus because of its role in metabolic homeostasis. In recent years, difficulties with peptide therapies have driven the search for small-molecule compounds to modulate the activity of this receptor. We recently identified quercetin, a naturally occurring flavonoid, as a probe-dependent, pathway-selective allosteric modulator of GLP-1R-mediated signaling. Using Chinese hamster ovary cells expressing the human GLP-1R, we have now extended this work to identify the structural requirements of flavonoids to modify GLP-1R binding and signaling (cAMP formation and intracellular Ca(2+) mobilization) of each of the GLP-1R endogenous agonists, as well as the clinically used exogenous peptide mimetic exendin-4. This study identified a chemical series of hydroxyl flavonols with the ability to selectively augment calcium (Ca(2+)) signaling in a peptide agonist-specific manner, with effects only on truncated GLP-1 peptides [GLP-1(7-36)NH(2) and GLP-1(7-37)] and exendin-4, but not on oxyntomodulin or full-length GLP-1 peptides [GLP-1(1-36)NH(2) and GLP-1(1-37)]. In addition, the 3-hydroxyl group on the flavone backbone (i.e., a flavonol) was essential for this activity, however insufficient on its own, to produce the allosteric effects. In contrast to hydroxyl flavonols, catechin had no effect on peptide-mediated Ca(2+) signaling but negatively modulated peptide-mediated cAMP formation in a probe-dependent manner. These data represent a detailed examination of the action of different flavonoids on peptide agonists at the GLP-1R and may aid in the development of future small molecule compounds targeted at this receptor.