The EMBL-EBI ChEMBL is a manually curated database of bioactive molecules with drug-like properties, either approved for marketing by the U.S Food and Drug Administration (FDA), or clinical candidates. ChEMBL also captures information regarding the drug molecule indications, as well as their curated pharmacological target.
In the Platform, ChEMBL evidence represents any target-disease relationship that can be explained by an approved or clinical candidate drug, targeting the gene product and indicated for the disease. Independent studies are treated as individual evidence.
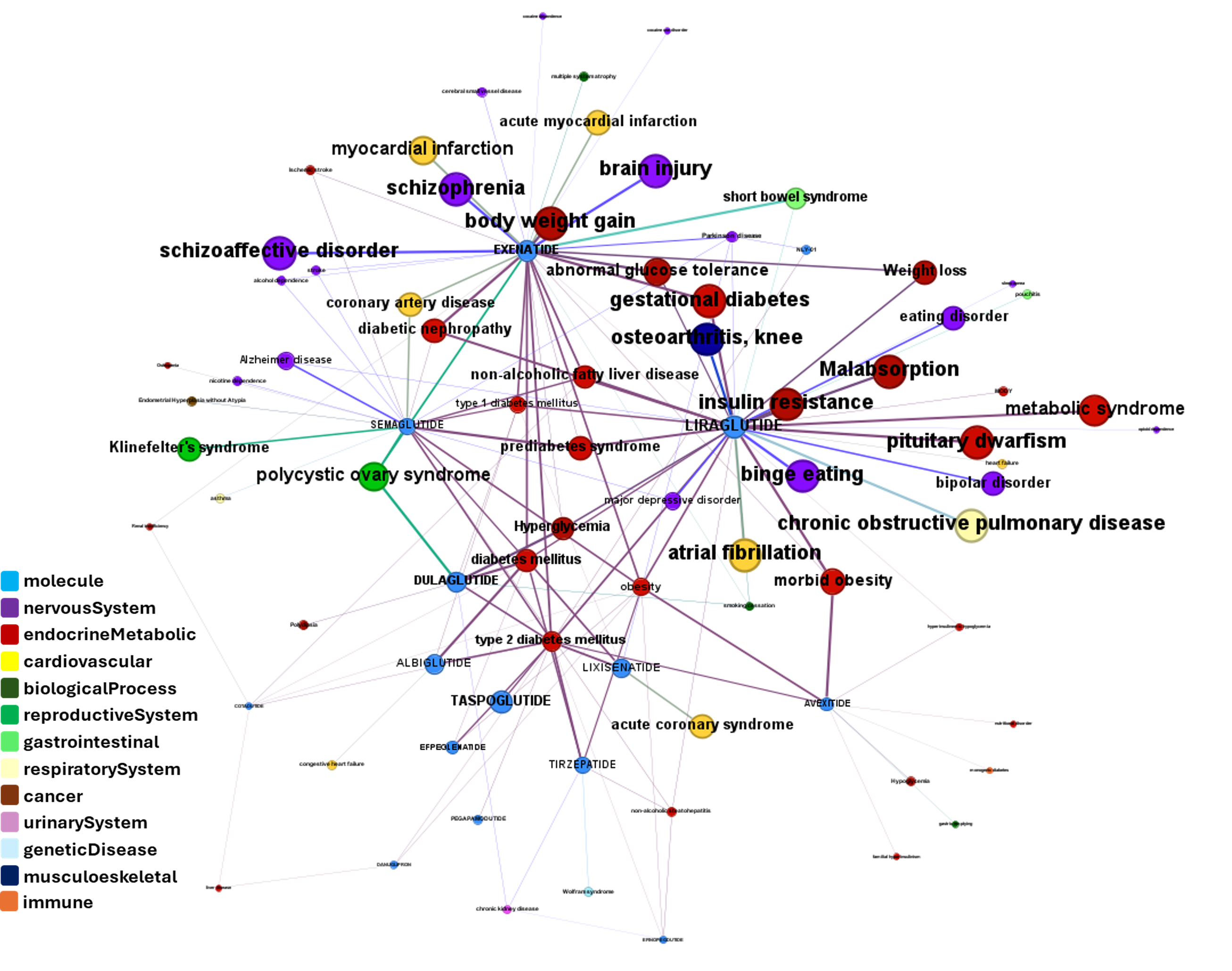
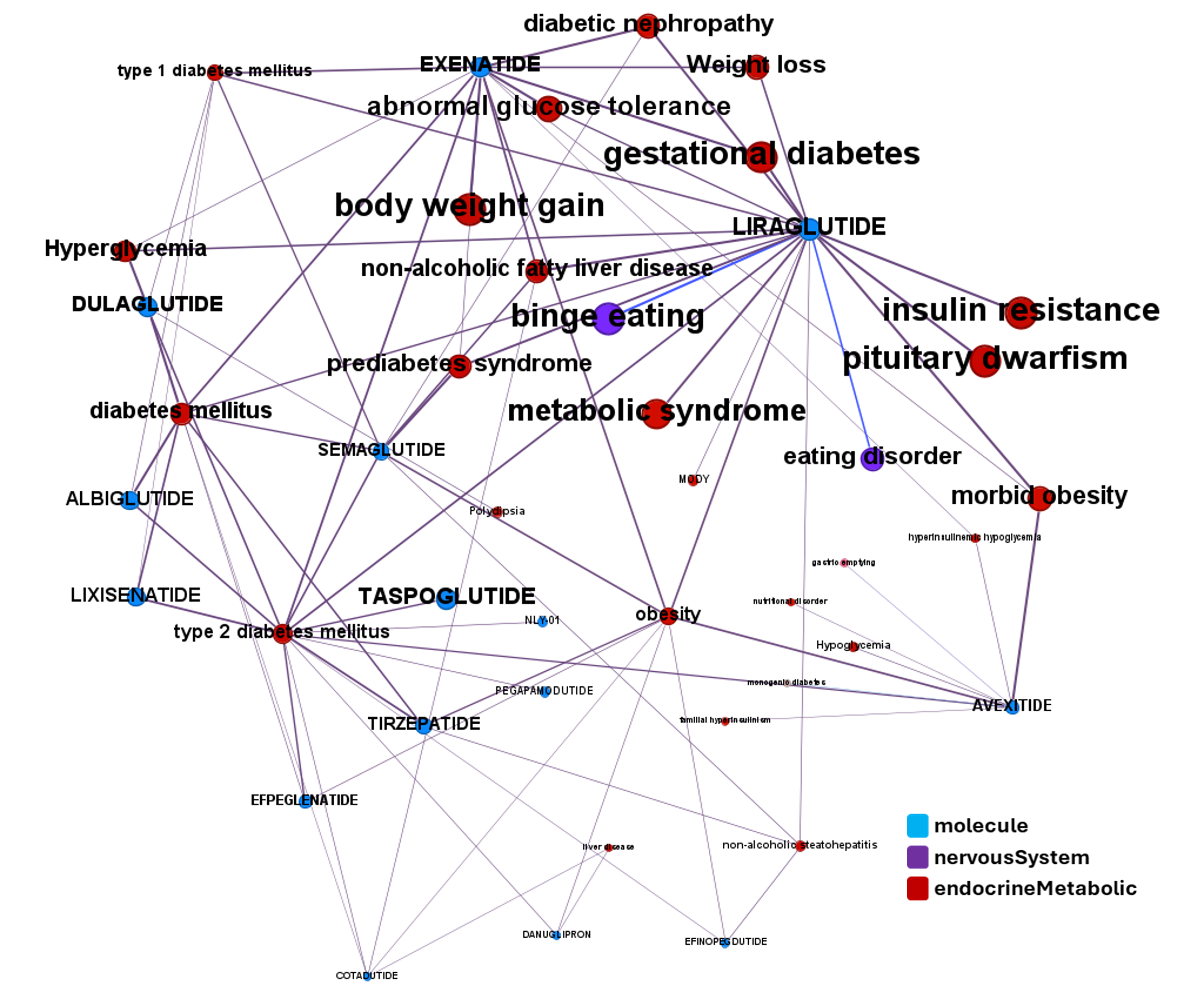
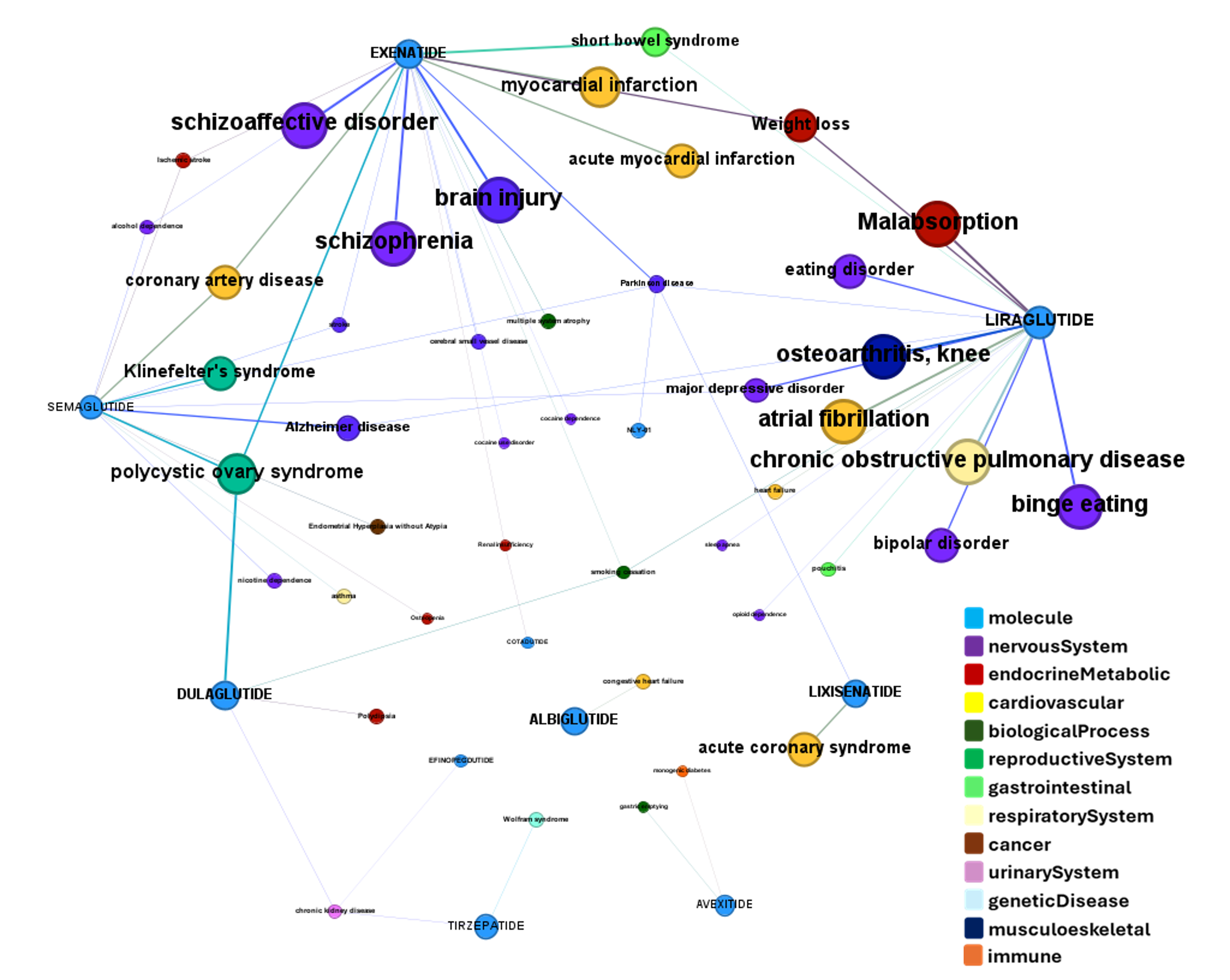
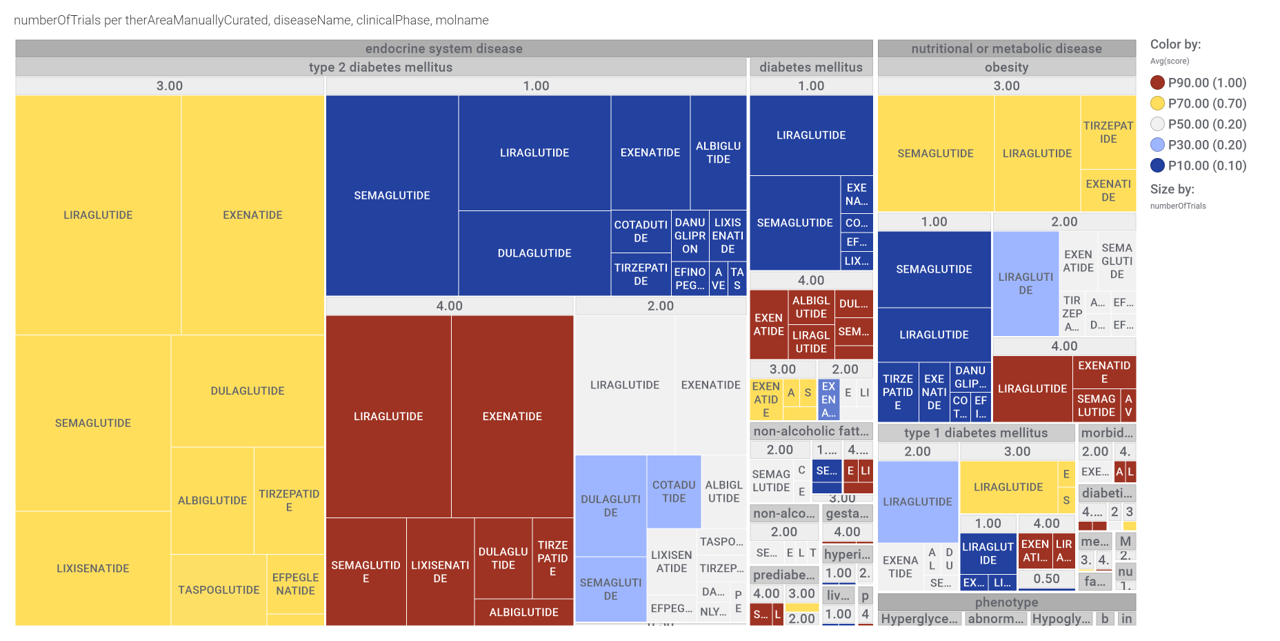
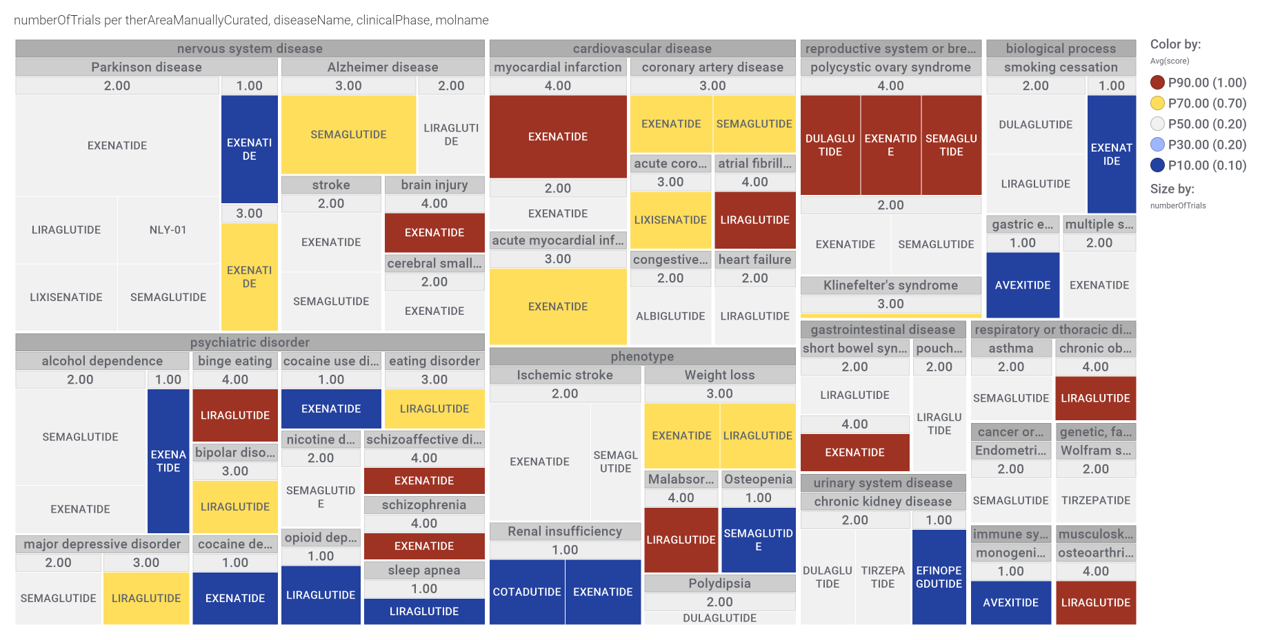
Here, a graph view of GLP1R association to diseases
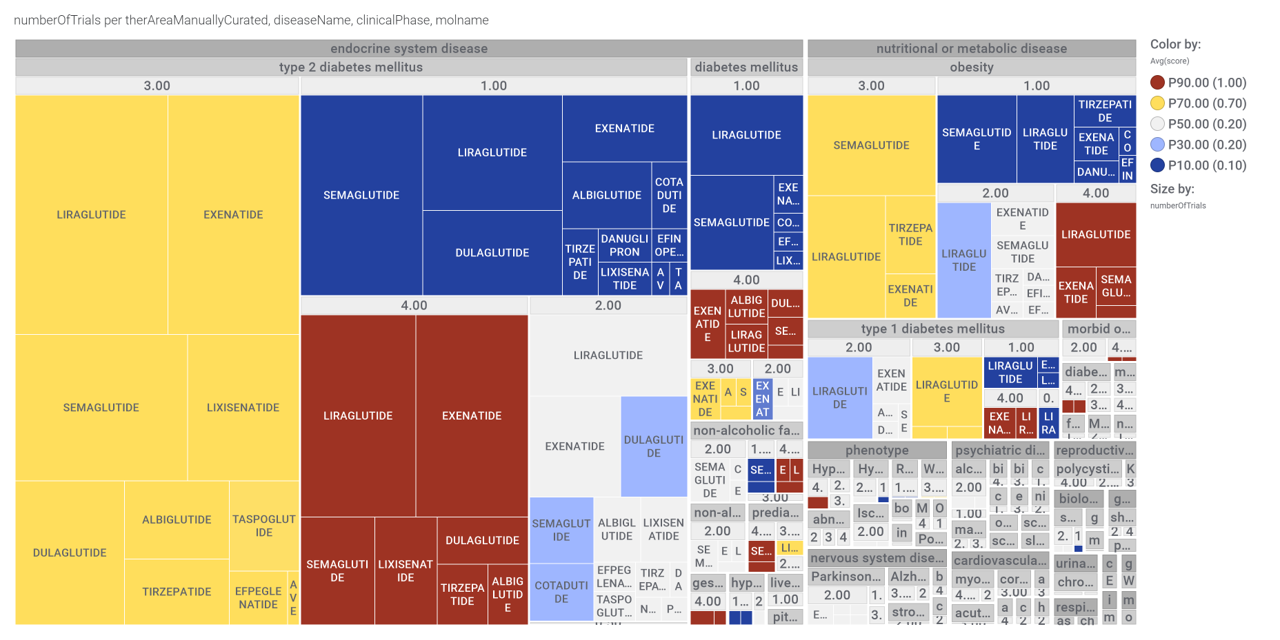
Disease associations table for GLP-1 receptors from clinical trials stored in ChEMBL and scored by OpenTargets.
| disease | molecule | therapeuticArea | associationScore | clinicalPhase | clinicalStatus | directionOnTrait | studyStartDate | studyStopReason | variantEffect | linkToClinicalTrial | linkToDisease |
|---|---|---|---|---|---|---|---|---|---|---|---|
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 2/20/2018 | Insufficient enrollment | GoF | https://clinicaltrials.gov/ct2/show/NCT03434119 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Withdrawn | protect | 3/9/2020 | COVID-19 | GoF | https://clinicaltrials.gov/ct2/show/NCT04287179 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 5/1/2013 | Decision by Sponsor | GoF | https://clinicaltrials.gov/ct2/show/NCT01785771 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 6/1/2012 | Difficulty recruiting patients | GoF | https://clinicaltrials.gov/ct2/show/NCT01597531 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| non-alcoholic fatty liver disease | COTADUTIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 1/3/2023 | Discontinuing the development of cotadutide, a daily injectable GLP-1/glucagon co-agonist, is\r based on strategic pipeline considerations. The premature closure is not due to any newly\r observed safety signals or a change in the risk/benefit profile. | GoF | https://clinicaltrials.gov/ct2/show/NCT05668936 | http://www.ebi.ac.uk/efo/EFO_0003095 |
| liver disease | COTADUTIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 9/6/2022 | Discontinuing the development of cotadutide, a daily injectable GLP-1/glucagon co-agonist, is\r based on strategic pipeline considerations.\r The premature closure is not due to any newly observed safety signals or a change in the\r risk/benefit profile. | GoF | https://clinicaltrials.gov/ct2/show/NCT05517226 | http://www.ebi.ac.uk/efo/EFO_0001421 |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Suspended | protect | 7/1/2023 | Due to an international shortage of Semaglutide, this study has been put on hold until the\r study drug is available again. | GoF | https://clinicaltrials.gov/ct2/show/NCT05424003 | http://www.ebi.ac.uk/efo/EFO_0003095 |
| alcohol dependence | EXENATIDE | psychiatric disorder | 0.1 | 1 | Terminated | protect | 5/2/2019 | Due to COVID-19 hospital-wide policies halting recruitment | GoF | https://clinicaltrials.gov/ct2/show/NCT03645408 | http://purl.obolibrary.org/obo/MONDO_0007079 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Terminated | protect | 9/26/2016 | Early termination due to insufficient enrollment. | GoF | https://clinicaltrials.gov/ct2/show/NCT02793154 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 9/26/2016 | Early termination due to insufficient enrollment. | GoF | https://clinicaltrials.gov/ct2/show/NCT02793154 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 2 | Terminated | protect | 8/1/2008 | Enrollment was much slower than anticipated, leading to a decision to terminate the study early for enrollment futility. | GoF | https://clinicaltrials.gov/ct2/show/NCT00701935 | http://www.ebi.ac.uk/efo/EFO_0000400 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 8/1/2012 | Error made by local pharmacy caused mixed randomization of 20 participants | GoF | https://clinicaltrials.gov/ct2/show/NCT01628445 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.35 | 3 | Terminated | protect | 1/1/2010 | high discontinuation rates mainly due to GI tolerability and implementation of risk mitigation plan to address hypersensitivity reactions | GoF | https://clinicaltrials.gov/ct2/show/NCT01051011 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Withdrawn | protect | 6/1/2016 | Inability to enroll due to the widespread use of both classes of drugs in patients with T2DM, including those on concomitant insulin therapy. | GoF | https://clinicaltrials.gov/ct2/show/NCT02811484 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Terminated | protect | 7/12/2016 | Insufficient recruitment rate | GoF | https://clinicaltrials.gov/ct2/show/NCT02767596 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Withdrawn | protect | 12/1/2019 | Investigator decided not to move forward with study prior to study start date | GoF | https://clinicaltrials.gov/ct2/show/NCT02198209 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.05 | 1 | Terminated | protect | 12/1/2013 | Lack of Efficacy | GoF | https://clinicaltrials.gov/ct2/show/NCT02020616 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Withdrawn | protect | 9/1/2020 | Lack of funds to cover the costs of the study medications | GoF | https://clinicaltrials.gov/ct2/show/NCT02920190 | http://www.ebi.ac.uk/efo/EFO_0001073 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Withdrawn | protect | 5/1/2012 | Lack of patients with the criteria established in the protocol. | GoF | https://clinicaltrials.gov/ct2/show/NCT01593137 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EFINOPEGDUTIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 7/12/2016 | manufacturing-related issues | GoF | https://clinicaltrials.gov/ct2/show/NCT02862431 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Withdrawn | protect | 11/1/2012 | No Funding Received from ADA | GoF | https://clinicaltrials.gov/ct2/show/NCT01722227 | http://purl.obolibrary.org/obo/MONDO_0005147 |
| short bowel syndrome | LIRAGLUTIDE | gastrointestinal disease | 0.2 | 2 | Withdrawn | protect | 10/20/2017 | No participants recruited. Not able to recruit due to COVID 19. | GoF | https://clinicaltrials.gov/ct2/show/NCT03371862 | http://purl.obolibrary.org/obo/MONDO_0015183 |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 8/25/2021 | Poor enrollment | GoF | https://clinicaltrials.gov/ct2/show/NCT04624672 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 1/29/2022 | Principal Investigator no longer at study site. | GoF | https://clinicaltrials.gov/ct2/show/NCT04938388 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Terminated | protect | 12/1/2011 | Recruiting failure | GoF | https://clinicaltrials.gov/ct2/show/NCT02417103 | http://www.ebi.ac.uk/efo/EFO_0001073 |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Withdrawn | protect | 10/6/2022 | Sponsor decision | GoF | https://clinicaltrials.gov/ct2/show/NCT05444153 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.2 | 2 | Terminated | protect | 4/1/2015 | Sponsor decision not related to safety reasons | GoF | https://clinicaltrials.gov/ct2/show/NCT02274740 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 11/9/2018 | Sponsor decision to cancel TRIAL, not related to safety concern | GoF | https://clinicaltrials.gov/ct2/show/NCT03713684 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 8/1/2019 | Sponsor decision to cancel TRIAL, not related to safety concern | GoF | https://clinicaltrials.gov/ct2/show/NCT03770728 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 4/27/2018 | Sponsor decision to cancel TRIAL, not related to safety concern | GoF | https://clinicaltrials.gov/ct2/show/NCT03496298 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 9/26/2018 | Sponsor decision to cancel TRIAL, not related to safety concern. | GoF | https://clinicaltrials.gov/ct2/show/NCT03684642 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 9/26/2018 | Sponsor decision to cancel TRIAL, not related to safety concern. | GoF | https://clinicaltrials.gov/ct2/show/NCT03684642 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| familial hyperinsulinism | AVEXITIDE | nutritional or metabolic disease | 0.1 | 1 | Terminated | protect | 8/26/2009 | Study stopped prematurely to test a new formulation of Exendin (9-39) | LoF | https://clinicaltrials.gov/ct2/show/NCT00835328 | http://purl.obolibrary.org/obo/MONDO_0017182 |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Withdrawn | protect | 8/1/2015 | Study was cancelled prior to enrolling any patients | GoF | https://clinicaltrials.gov/ct2/show/NCT02229240 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| short bowel syndrome | EXENATIDE | gastrointestinal disease | 1 | 4 | Withdrawn | protect | 3/1/2013 | Study was withdrawn by PI due to decision to study a different medication. | GoF | https://clinicaltrials.gov/ct2/show/NCT01818648 | http://purl.obolibrary.org/obo/MONDO_0015183 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 10/1/2012 | Terminated because of insufficient number of subjects included. | GoF | https://clinicaltrials.gov/ct2/show/NCT01638260 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Terminated | protect | 3/21/2012 | The decision to close the NN2211-3619 trial was based on the very low recruitment rate as well as challenges relating to trial execution and study completion. | GoF | https://clinicaltrials.gov/ct2/show/NCT01206101 | http://purl.obolibrary.org/obo/MONDO_0005147 |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Withdrawn | protect | 12/1/2017 | The sponsor decides withdrawn this study. | GoF | https://clinicaltrials.gov/ct2/show/NCT04839237 | http://www.ebi.ac.uk/efo/EFO_0001073 |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Withdrawn | protect | 8/14/2017 | The study did not start recruiting as albiglutide would have been withdrawn from the market prior to study end. | GoF | https://clinicaltrials.gov/ct2/show/NCT03015519 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| eating disorder | LIRAGLUTIDE | psychiatric disorder | 0.7 | 3 | Terminated | protect | 9/29/2017 | The study was not meeting recruitment goals. | GoF | https://clinicaltrials.gov/ct2/show/NCT03279731 | http://www.ebi.ac.uk/efo/EFO_0005203 |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 10/7/2016 | The termination was result of GSK business considerations and not due to quality, safety or efficacy concerns with any albiglutide formulations or study conduct | GoF | https://clinicaltrials.gov/ct2/show/NCT02750930 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Terminated | protect | 2/1/2006 | The trial was terminated at week 195 due to an insufficient number of subjects remaining to obtain reasonable statistical power | GoF | https://clinicaltrials.gov/ct2/show/NCT00294723 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Terminated | protect | 7/15/2019 | The trial was terminated for strategic reasons. | GoF | https://clinicaltrials.gov/ct2/show/NCT04012255 | http://www.ebi.ac.uk/efo/EFO_0001073 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Terminated | protect | 2/1/2014 | The trial was terminated per protocol because of lack of feasibility. | GoF | https://clinicaltrials.gov/ct2/show/NCT02072096 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 2/1/2014 | The trial was terminated per protocol because of lack of feasibility. | GoF | https://clinicaltrials.gov/ct2/show/NCT02072096 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 7/16/2015 | Too challenging to recruit appropriate participants at an acceptable speed. | GoF | https://clinicaltrials.gov/ct2/show/NCT02231658 | http://www.ebi.ac.uk/efo/EFO_0000400 |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 7/16/2015 | Too challenging to recruit appropriate participants at an acceptable speed. | GoF | https://clinicaltrials.gov/ct2/show/NCT02231658 | http://www.ebi.ac.uk/efo/EFO_0000400 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Terminated | protect | 2/1/2011 | unavailability of study drug and matching placebo | GoF | https://clinicaltrials.gov/ct2/show/NCT01381926 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Terminated | protect | 6/1/2016 | We concluded that it would not be possible to meet our recruitment targets within the available\r budget. The study was ended when the funding ended. | GoF | https://clinicaltrials.gov/ct2/show/NCT05762744 | http://purl.obolibrary.org/obo/MONDO_0005148 |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00717457 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00254800 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00603239 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00577824 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00612794 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00359879 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/2/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01885208 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01798264 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 9/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02455076 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 10/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00241423 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00641056 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01064687 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02288273 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT00085969 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02380521 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/2/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01554618 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02787551 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/29/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01676116 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00637273 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00313001 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00948168 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/8/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT03059719 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c621d002-b7bf-4f3a-af68-4f70fd298d05 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/18/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01144338 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00254254 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01003184 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00044694 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 2/28/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03331289 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT01789957 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02325960 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01104701 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT00099320 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02092597 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00375492 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00135330 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00324363 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00111540 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00516074 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=71fe88be-b4e6-4c2d-9cc3-8b1864467776 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00943917 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00308139 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00039013 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00676338 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT00747968 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 3/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00870194 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00894322 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT01154933 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 11/17/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03444142 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT00099619 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01776788 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01652716 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01664624 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT00099333 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00964262 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02162550 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02119819 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 3/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00667732 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00434954 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00517283 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01524705 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00035984 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 12/15/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02981069 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00039026 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Not yet recruiting | protect | 9/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05670379 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00359762 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02194595 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT00082407 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/28/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02533453 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT00082381 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2d18cfc4-e0de-4814-a712-c1b7c504bff5 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01744236 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00917267 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53d03c03-ebf7-418d-88a8-533eabd2ee4f | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT00044668 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00753896 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00516048 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 11/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01270191 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01951651 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01029886 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT01876849 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00508287 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01181986 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00381342 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00877890 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00518882 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00360334 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/30/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00658021 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00353834 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00960661 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00103935 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT00097500 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00950677 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00971659 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00729326 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 2/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT01435980 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02129985 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 5/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00477581 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00765817 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00382239 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Not yet recruiting | protect | 2/1/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03018665 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 11/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02449603 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00707031 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01843127 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00259896 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00518115 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1c8ee2a-6a76-435e-ad38-8a9c99046ad9 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 6/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT01432405 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Unknown status | protect | 11/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01140893 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00935532 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| schizophrenia | EXENATIDE | psychiatric disorder | 1 | 4 | Completed | protect | 9/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02417142 | http://purl.obolibrary.org/obo/MONDO_0005090 | |
| alcohol dependence | EXENATIDE | psychiatric disorder | 0.2 | 2 | Completed | protect | 8/7/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03232112 | http://purl.obolibrary.org/obo/MONDO_0007079 | |
| abnormal glucose tolerance | EXENATIDE | phenotype | 1 | 4 | Completed | protect | 10/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00546728 | http://www.ebi.ac.uk/efo/EFO_0002546 | |
| smoking cessation | EXENATIDE | biological process | 0.1 | 1 | Completed | protect | 7/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02975297 | http://www.ebi.ac.uk/efo/EFO_0004319 | |
| cerebral small vessel disease | EXENATIDE | nervous system disease | 0.2 | 2 | Recruiting | protect | 5/25/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05356104 | http://www.ebi.ac.uk/efo/EFO_0008493 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01652729 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Terminated | protect | 9/20/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02802514 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01156779 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/6/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02229383 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ01 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT01076842 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 7/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT00064714 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT02042664 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/24/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03167411 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01107717 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT03297879 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| Renal insufficiency | EXENATIDE | phenotype | 0.1 | 1 | Completed | protect | 11/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02320045 | http://purl.obolibrary.org/obo/HP_0000083 | |
| non-alcoholic fatty liver disease | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00650546 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | EXENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT01208649 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| morbid obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 5/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00886626 | http://www.ebi.ac.uk/efo/EFO_0001074 | |
| morbid obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 12/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02496611 | http://www.ebi.ac.uk/efo/EFO_0001074 | |
| morbid obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Unknown status | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00872378 | http://www.ebi.ac.uk/efo/EFO_0001074 | |
| diabetic nephropathy | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 4/8/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02690883 | http://www.ebi.ac.uk/efo/EFO_0000401 | |
| myocardial infarction | EXENATIDE | cardiovascular disease | 0.2 | 2 | Unknown status | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01938235 | http://www.ebi.ac.uk/efo/EFO_0000612 | |
| myocardial infarction | EXENATIDE | cardiovascular disease | 1 | 4 | Completed | protect | 9/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01580514 | http://www.ebi.ac.uk/efo/EFO_0000612 | |
| myocardial infarction | EXENATIDE | cardiovascular disease | 1 | 4 | Completed | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00835848 | http://www.ebi.ac.uk/efo/EFO_0000612 | |
| stroke | EXENATIDE | nervous system disease | 0.2 | 2 | Completed | protect | 8/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02838589 | http://www.ebi.ac.uk/efo/EFO_0000712 | |
| brain injury | EXENATIDE | nervous system disease | 1 | 4 | Completed | protect | 8/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02058940 | http://purl.obolibrary.org/obo/MONDO_0043510 | |
| Hyperglycemia | EXENATIDE | phenotype | 0.2 | 2 | Completed | protect | 1/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT01969149 | http://purl.obolibrary.org/obo/HP_0003074 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 12/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT03017352 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.7 | 3 | Enrolling by invitation | protect | 8/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00923715 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00456300 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 12/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01269034 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Unknown status | protect | 7/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02584582 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 9/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01928329 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 8/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01269047 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 1/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01855490 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 11/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01235819 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| cocaine use disorder | EXENATIDE | psychiatric disorder | 0.1 | 1 | Completed | protect | 6/24/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04941521 | http://www.ebi.ac.uk/efo/EFO_0010445 | |
| schizoaffective disorder | EXENATIDE | psychiatric disorder | 1 | 4 | Completed | protect | 9/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02417142 | http://www.ebi.ac.uk/efo/EFO_0005411 | |
| acute myocardial infarction | EXENATIDE | cardiovascular disease | 0.7 | 3 | Unknown status | protect | 11/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01254123 | http://www.ebi.ac.uk/efo/EFO_0008583 | |
| acute myocardial infarction | EXENATIDE | cardiovascular disease | 0.7 | 3 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02404376 | http://www.ebi.ac.uk/efo/EFO_0008583 | |
| multiple system atrophy | EXENATIDE | biological process | 0.2 | 2 | Recruiting | protect | 9/16/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04431713 | http://www.ebi.ac.uk/efo/EFO_1001050 | |
| cocaine dependence | EXENATIDE | psychiatric disorder | 0.1 | 1 | Completed | protect | 11/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02302976 | http://www.ebi.ac.uk/efo/EFO_0002610 | |
| gestational diabetes | EXENATIDE | endocrine system disease | 1 | 4 | Withdrawn | protect | 8/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT00572689 | http://www.ebi.ac.uk/efo/EFO_0004593 | |
| gestational diabetes | EXENATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 4/12/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05482789 | http://www.ebi.ac.uk/efo/EFO_0004593 | |
| coronary artery disease | EXENATIDE | cardiovascular disease | 0.7 | 3 | Completed | protect | 6/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01373216 | http://www.ebi.ac.uk/efo/EFO_0001645 | |
| Ischemic stroke | EXENATIDE | phenotype | 0.2 | 2 | Recruiting | protect | 8/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02829502 | http://purl.obolibrary.org/obo/HP_0002140 | |
| Ischemic stroke | EXENATIDE | phenotype | 0.2 | 2 | Active, not recruiting | protect | 11/23/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03287076 | http://purl.obolibrary.org/obo/HP_0002140 | |
| Parkinson disease | EXENATIDE | nervous system disease | 0.2 | 2 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01971242 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| Parkinson disease | EXENATIDE | nervous system disease | 0.2 | 2 | Active, not recruiting | protect | 1/21/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04305002 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| Parkinson disease | EXENATIDE | nervous system disease | 0.7 | 3 | Active, not recruiting | protect | 1/20/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04232969 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| Parkinson disease | EXENATIDE | nervous system disease | 0.1 | 1 | Completed | protect | 6/5/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03456687 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| Parkinson disease | EXENATIDE | nervous system disease | 0.2 | 2 | Unknown status | protect | 7/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01174810 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| prediabetes syndrome | EXENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/9/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03961256 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| Weight loss | EXENATIDE | phenotype | 0.7 | 3 | Completed | protect | 3/3/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00856609 | http://purl.obolibrary.org/obo/HP_0001824 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Unknown status | protect | 8/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT03002675 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 9/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02794402 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 9/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03671733 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 1/28/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04520490 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 10/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01237197 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 1/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01061775 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/3/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00856609 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 3/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01590433 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02170324 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02160990 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 12/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01501084 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 5/16/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01857895 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 12/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02313220 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 6/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00500370 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EXENATIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02664441 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| hyperinsulinemic hypoglycemia | EXENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02685852 | http://www.ebi.ac.uk/efo/EFO_0007318 | |
| polycystic ovary syndrome | EXENATIDE | reproductive system or breast disease | 0.2 | 2 | Completed | protect | 6/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00344851 | http://www.ebi.ac.uk/efo/EFO_0000660 | |
| polycystic ovary syndrome | EXENATIDE | reproductive system or breast disease | 1 | 4 | Completed | protect | 1/4/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04969627 | http://www.ebi.ac.uk/efo/EFO_0000660 | |
| body weight gain | EXENATIDE | phenotype | 1 | 4 | Completed | protect | 12/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00845507 | http://www.ebi.ac.uk/efo/EFO_0004566 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/15/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01077505 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01098461 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00838903 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02787551 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fcad939-76e7-49cf-af94-4e6aef17901f | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 6/11/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02496221 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00849017 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/16/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02683746 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00518115 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 7/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01357889 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/6/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01406262 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/11/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01147718 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00839527 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00838916 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00849056 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01128894 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/19/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01147692 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01733758 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01475734 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/21/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02229227 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02660736 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/16/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00394030 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00354536 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01098539 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/4/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01147731 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00530309 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/5/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00938158 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/7/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00537719 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ04 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | Terminated | protect | 9/20/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02802514 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/23/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01777282 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | ALBIGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02465515 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| type 1 diabetes mellitus | ALBIGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 10/10/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02284009 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| congestive heart failure | ALBIGLUTIDE | cardiovascular disease | 0.2 | 2 | Completed | protect | 9/15/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01357850 | http://www.ebi.ac.uk/efo/EFO_0000373 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00423501 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00809705 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00744367 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00744926 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00755287 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01018173 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00909597 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00754988 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00811460 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00823992 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00460941 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TASPOGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00717457 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/25/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04641312 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01468181 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01191268 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/7/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04591626 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 11/30/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05564039 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01215968 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 10/12/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05516966 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01458210 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01524770 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/18/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04143802 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01624259 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/25/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04515576 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02152371 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00791479 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/31/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05459285 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/13/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04809220 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 6/2/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05377333 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/29/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02963766 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01064687 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 5/26/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04893148 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00630825 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02973100 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 6/7/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05407961 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Not yet recruiting | protect | 6/15/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05348122 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01149421 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/7/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03861052 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 10/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01001104 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/11/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02759107 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02597049 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01432938 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02787551 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 12/29/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05659537 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00734474 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/15/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05048719 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=463050bd-2b1c-40f5-b3c3-0a04bb433309 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/5/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03495102 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02648204 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01324388 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/13/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04867785 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 5/29/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04255433 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 2/9/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05680129 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01584232 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01558271 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01408888 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01769378 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01253304 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/28/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03315780 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01436201 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Active, not recruiting | protect | 8/6/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04965506 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01300260 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01075282 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01301092 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/10/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03015220 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 11/17/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03444142 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/24/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03131687 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/12/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04466904 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Not yet recruiting | protect | 11/23/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05606913 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01126580 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02750410 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01667900 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01648582 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DULAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01644500 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| Polydipsia | DULAGLUTIDE | phenotype | 0.2 | 2 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02770885 | http://purl.obolibrary.org/obo/HP_0001959 | |
| smoking cessation | DULAGLUTIDE | biological process | 0.2 | 2 | Completed | protect | 6/26/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03204396 | http://www.ebi.ac.uk/efo/EFO_0004319 | |
| diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ05 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | DULAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03824002 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| Hyperglycemia | DULAGLUTIDE | phenotype | 1 | 4 | Recruiting | protect | 3/8/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03743025 | http://purl.obolibrary.org/obo/HP_0003074 | |
| Hyperglycemia | DULAGLUTIDE | phenotype | 1 | 4 | Recruiting | protect | 7/29/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04862234 | http://purl.obolibrary.org/obo/HP_0003074 | |
| type 1 diabetes mellitus | DULAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 1/31/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03668470 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| chronic kidney disease | DULAGLUTIDE | urinary system disease | 0.2 | 2 | Recruiting | protect | 3/15/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05254418 | http://www.ebi.ac.uk/efo/EFO_0003884 | |
| polycystic ovary syndrome | DULAGLUTIDE | reproductive system or breast disease | 1 | 4 | Completed | protect | 5/15/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04876027 | http://www.ebi.ac.uk/efo/EFO_0000660 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/17/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02803918 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01517412 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02200991 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00975286 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02020629 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00715624 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/27/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05114590 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 7/7/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05413369 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01940965 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01572649 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1727cc16-4f86-4f13-b8b5-804d4984fa8c | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02049034 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01169779 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471Orig1s000lbl.pdf | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/9/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02749890 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 2/23/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02941367 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/11/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03767543 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02058147 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01175473 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bba538b-cf7c-4310-ae8f-cb711ed21bcc | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01798706 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00707031 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01596504 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00299871 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01960179 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00931372 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00712673 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02787551 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00976937 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00763451 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00713830 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01768559 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01632163 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00905255 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 11/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01476475 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00763815 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01973231 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/15/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03798054 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02168491 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00688701 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/2/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03819790 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIXISENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00866658 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| diabetes mellitus | LIXISENATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ03 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| type 1 diabetes mellitus | LIXISENATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 6/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01146678 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| Parkinson disease | LIXISENATIDE | nervous system disease | 0.2 | 2 | Completed | protect | 6/13/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03439943 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| acute coronary syndrome | LIXISENATIDE | cardiovascular disease | 0.7 | 3 | Completed | protect | 6/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01147250 | http://www.ebi.ac.uk/efo/EFO_0005672 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/23/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03598621 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/30/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03987919 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 6/17/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03914326 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 11/2/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04596631 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/21/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02146079 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/21/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03288740 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/29/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04524832 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 4/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05259033 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/17/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03789578 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/8/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03611322 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03689374 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/18/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03724981 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Recruiting | protect | 8/8/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05486065 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Active, not recruiting | protect | 8/12/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04032197 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 11/29/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05144984 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/19/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01686945 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 6/22/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05435677 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/10/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03015220 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/2/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04109508 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05498610 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/20/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02849080 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=27f15fac-7d98-4114-a2ec-92494a91da98 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/28/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03951753 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02161588 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/2/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01885208 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/17/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03638778 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04109547 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/2/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02254291 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 8/3/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05478252 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/20/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02827708 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 7/25/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05816057 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/20/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02906930 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01324505 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/2/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02773381 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/27/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03191396 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/15/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03136484 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 1/17/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05689099 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 3/29/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05533632 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/12/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01572753 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/15/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02607865 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 1/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05597202 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/28/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03061214 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/21/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02147431 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/10/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02863419 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/11/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02212067 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 3/13/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05441267 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/11/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT03144271 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/10/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02863328 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02648204 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02845219 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/15/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04707469 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/17/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02022254 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/8/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04016974 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 5/8/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03811561 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/10/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03018028 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/21/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01720446 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/21/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02461589 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 9/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05035082 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Not yet recruiting | protect | 9/5/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05813912 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/4/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02128932 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 8/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04126603 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/19/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05232708 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/22/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02877355 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/2/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03021187 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/25/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04152915 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01866748 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/17/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02692716 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02305381 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/2/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04982575 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/15/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03466567 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/3/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05129891 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 3/3/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05303857 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 7/13/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03596450 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/13/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05153564 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/29/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04017832 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00813020 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/6/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05046873 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 9/10/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04817644 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/4/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02207374 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/30/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04153929 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/2/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01923181 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/28/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04513704 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/12/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02016911 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.05 | 0.5 | Recruiting | protect | 3/10/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05249881 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Active, not recruiting | protect | 2/4/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05227196 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 1/18/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04538352 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/11/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02014259 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/15/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03086330 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/18/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04878406 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/3/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02054897 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 6/17/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03819153 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/19/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03989232 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/3/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00696657 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5399d16-01c1-4315-af3c-7619fee86621 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/30/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04097600 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01272973 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/2/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01930188 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| alcohol dependence | SEMAGLUTIDE | psychiatric disorder | 0.2 | 2 | Recruiting | protect | 6/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05891587 | http://purl.obolibrary.org/obo/MONDO_0007079 | |
| alcohol dependence | SEMAGLUTIDE | psychiatric disorder | 0.2 | 2 | Not yet recruiting | protect | 7/15/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05892432 | http://purl.obolibrary.org/obo/MONDO_0007079 | |
| Alzheimer disease | SEMAGLUTIDE | nervous system disease | 0.7 | 3 | Recruiting | protect | 6/20/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05891496 | http://purl.obolibrary.org/obo/MONDO_0004975 | |
| Alzheimer disease | SEMAGLUTIDE | nervous system disease | 0.7 | 3 | Recruiting | protect | 5/18/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04777409 | http://purl.obolibrary.org/obo/MONDO_0004975 | |
| asthma | SEMAGLUTIDE | respiratory or thoracic disease | 0.2 | 2 | Recruiting | protect | 10/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05254314 | http://purl.obolibrary.org/obo/MONDO_0004979 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 2/17/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT04854083 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01766245 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02231684 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02172313 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/24/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02249871 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Active, not recruiting | protect | 9/10/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04451837 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/26/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02064348 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00851773 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ06 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/10/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02920385 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01619345 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/16/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02243098 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/4/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02060266 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 7/12/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05305794 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02557620 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/5/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03010475 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/27/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02070510 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/18/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02249910 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 2/15/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05813249 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/28/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03357380 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 4/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04822181 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 11/30/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02970942 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Recruiting | protect | 8/31/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05016882 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.1 | 1 | Recruiting | protect | 3/2/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05766709 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/18/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03987451 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| non-alcoholic fatty liver disease | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Recruiting | protect | 7/24/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03884075 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| major depressive disorder | SEMAGLUTIDE | psychiatric disorder | 0.2 | 2 | Recruiting | protect | 10/6/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04466345 | http://purl.obolibrary.org/obo/MONDO_0002009 | |
| nicotine dependence | SEMAGLUTIDE | psychiatric disorder | 0.2 | 2 | Recruiting | protect | 10/7/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05530577 | http://www.ebi.ac.uk/efo/EFO_0003768 | |
| diabetic nephropathy | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 6/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05897372 | http://www.ebi.ac.uk/efo/EFO_0000401 | |
| stroke | SEMAGLUTIDE | nervous system disease | 0.2 | 2 | Recruiting | protect | 4/23/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05920889 | http://www.ebi.ac.uk/efo/EFO_0000712 | |
| Klinefelter's syndrome | SEMAGLUTIDE | reproductive system or breast disease | 0.7 | 3 | Recruiting | protect | 3/21/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05586802 | http://www.ebi.ac.uk/efo/EFO_1001006 | |
| type 1 diabetes mellitus | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 5/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03899402 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 6/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05819138 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| Osteopenia | SEMAGLUTIDE | phenotype | 0.1 | 1 | Recruiting | protect | 3/24/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04702516 | http://purl.obolibrary.org/obo/HP_0000938 | |
| coronary artery disease | SEMAGLUTIDE | cardiovascular disease | 0.7 | 3 | Not yet recruiting | protect | 11/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05071417 | http://www.ebi.ac.uk/efo/EFO_0001645 | |
| Ischemic stroke | SEMAGLUTIDE | phenotype | 0.2 | 2 | Recruiting | protect | 4/12/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05630586 | http://purl.obolibrary.org/obo/HP_0002140 | |
| Parkinson disease | SEMAGLUTIDE | nervous system disease | 0.2 | 2 | Not yet recruiting | protect | 1/2/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03659682 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| prediabetes syndrome | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 1/13/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT04873050 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| prediabetes syndrome | SEMAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 11/22/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05119179 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| non-alcoholic steatohepatitis | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Recruiting | protect | 8/9/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04971785 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| non-alcoholic steatohepatitis | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/4/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04944992 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| non-alcoholic steatohepatitis | SEMAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 7/29/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03987074 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 11/1/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05567796 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/3/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04238962 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 12/16/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04187300 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 11/16/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05132088 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 8/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03611582 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/16/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04788511 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 6/4/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03548987 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 3/15/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT04873245 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 4/3/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05813925 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 1/19/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05579249 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 1/4/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05646706 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Recruiting | protect | 3/15/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05751226 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 10/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05564117 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 9/6/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05040971 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 10/7/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04102189 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Not yet recruiting | protect | 8/3/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05202353 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Active, not recruiting | protect | 11/16/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05616013 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 7/26/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05548647 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 8/15/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT04998136 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 7/15/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04969939 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 1/29/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04228354 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 10/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02453711 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 6/18/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03574584 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 1/21/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03811574 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 5/30/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05890976 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/8/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05236517 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 9/11/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04074161 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/15/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03842202 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 7/25/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03600480 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 6/1/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05574439 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 6/4/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03552757 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 7/7/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04940078 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 10/5/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03693430 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 9/13/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05035095 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Recruiting | protect | 2/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04779697 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 4/21/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05822830 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Not yet recruiting | protect | 7/7/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05726227 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 3/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05669755 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 10/24/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03574597 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 6/4/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03548935 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 10/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05064735 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Active, not recruiting | protect | 1/19/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05671653 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | SEMAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 10/15/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03707990 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| Endometrial Hyperplasia without Atypia | SEMAGLUTIDE | cancer or benign tumor | 0.2 | 2 | Not yet recruiting | protect | 10/31/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05829460 | http://www.ebi.ac.uk/efo/EFO_1000234 | |
| polycystic ovary syndrome | SEMAGLUTIDE | reproductive system or breast disease | 1 | 4 | Not yet recruiting | protect | 4/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05702905 | http://www.ebi.ac.uk/efo/EFO_0000660 | |
| polycystic ovary syndrome | SEMAGLUTIDE | reproductive system or breast disease | 0.2 | 2 | Not yet recruiting | protect | 1/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05646199 | http://www.ebi.ac.uk/efo/EFO_0000660 | |
| type 2 diabetes mellitus | PEGAPAMODUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 4/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02119819 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00395746 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00393718 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01596504 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3946d389-0926-4f77-a708-0acb8153b143 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/26/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02420262 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 12/1/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05767255 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02638805 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/29/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01676116 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/18/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02205528 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03707171 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01779362 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/5/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01572740 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01511198 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 5/1/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04146155 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 7/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01516255 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01515592 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00614120 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01473953 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01270789 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/11/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01296412 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00154401 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/10/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03018028 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/17/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01037582 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/21/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02505334 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04657939 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/31/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03115099 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/2/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02973321 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01388361 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/27/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03191396 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | GoF | https://clinicaltrials.gov/ct2/show/NCT00013910 | http://purl.obolibrary.org/obo/MONDO_0005148 | ||
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00333151 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00856986 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01916174 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 10/1/2000 | GoF | https://clinicaltrials.gov/ct2/show/NCT01509755 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/14/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01620489 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01509742 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00873223 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/21/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT05029076 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01508858 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01517555 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 3/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01785043 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01733758 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/3/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02964247 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/13/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01541215 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/21/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02911948 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 5/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01931982 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/10/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02863419 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/23/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02773368 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01558271 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01208012 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT01511692 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/6/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02787551 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01373450 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01511185 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01658501 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02100475 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/9/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01917656 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT00154414 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01128894 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/2/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01907854 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01175473 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00331851 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01973231 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01117350 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT02721888 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01508923 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01937598 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01499108 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT01508806 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02832999 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/20/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01952145 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Terminated | protect | 11/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01516476 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00518882 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01029886 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/1999 | GoF | https://clinicaltrials.gov/ct2/show/NCT01507285 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00993304 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/20/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05360147 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/26/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03175120 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01664247 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Unknown status | protect | 6/20/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03421119 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Not yet recruiting | protect | 5/1/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04373967 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/26/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03449654 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01507389 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01790308 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02113332 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00318422 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/3/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00696657 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/21/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02461589 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/26/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03172494 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=21335fe4-d395-4501-ac2a-2f20d7520da9 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/1999 | GoF | https://clinicaltrials.gov/ct2/show/NCT01507311 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00943501 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05360537 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 5/18/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02686177 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Active, not recruiting | protect | 5/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02344186 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/23/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01336023 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/29/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03292185 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 2/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02765399 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 3/28/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02730377 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/9/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02960659 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 3/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01919489 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01870297 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02057172 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/31/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02501161 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01794143 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/31/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03555994 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/2/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03235050 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/22/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT05294536 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01664676 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5a9ef4ea-c76a-4d34-a604-27c5b505f5a4 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01624259 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 9/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01617434 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 2/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00620282 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT02292290 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT01511172 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 1/10/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01512108 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/31/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01179048 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/18/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02607306 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT00318461 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00700817 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01654120 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Unknown status | protect | 10/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02501850 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/1/2002 | GoF | https://clinicaltrials.gov/ct2/show/NCT01620463 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/29/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01618162 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/21/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02298192 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Terminated | protect | 7/27/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02492763 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/28/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01392573 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 10/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01455441 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 1/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01518101 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT01615978 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/4/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02889510 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 11/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01966978 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01744236 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 12/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02008682 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01508949 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Recruiting | protect | 1/24/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04535960 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7b11f6ac-4c27-4748-9e65-22d4cf3adfb4 | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| atrial fibrillation | LIRAGLUTIDE | cardiovascular disease | 1 | 4 | Active, not recruiting | protect | 3/18/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03856632 | http://www.ebi.ac.uk/efo/EFO_0000275 | |
| abnormal glucose tolerance | LIRAGLUTIDE | phenotype | 0.2 | 2 | Unknown status | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01845259 | http://www.ebi.ac.uk/efo/EFO_0002546 | |
| abnormal glucose tolerance | LIRAGLUTIDE | phenotype | 0.7 | 3 | Completed | protect | 8/11/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01234649 | http://www.ebi.ac.uk/efo/EFO_0002546 | |
| pituitary dwarfism | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Not yet recruiting | protect | 5/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05681299 | http://www.ebi.ac.uk/efo/EFO_1001109 | |
| smoking cessation | LIRAGLUTIDE | biological process | 0.2 | 2 | Completed | protect | 11/29/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03712098 | http://www.ebi.ac.uk/efo/EFO_0004319 | |
| heart failure | LIRAGLUTIDE | cardiovascular disease | 0.2 | 2 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01800968 | http://www.ebi.ac.uk/efo/EFO_0003144 | |
| Alzheimer disease | LIRAGLUTIDE | nervous system disease | 0.2 | 2 | Unknown status | protect | 1/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01843075 | http://purl.obolibrary.org/obo/MONDO_0004975 | |
| opioid dependence | LIRAGLUTIDE | psychiatric disorder | 0.1 | 1 | Recruiting | protect | 10/18/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04199728 | http://www.ebi.ac.uk/efo/EFO_0005611 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00761540 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01319240 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/7/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01982630 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Unknown status | protect | 12/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02059564 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/28/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT01508897 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/13/2005 | GoF | https://clinicaltrials.gov/ct2/show/NCT01514487 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/1/2004 | GoF | https://clinicaltrials.gov/ct2/show/NCT01507337 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 3/1/1999 | GoF | https://clinicaltrials.gov/ct2/show/NCT01507272 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2003 | GoF | https://clinicaltrials.gov/ct2/show/NCT01620476 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35e613e3-7aae-4152-b827-99d1ee7841be | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 2/28/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03883412 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT01513525 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01517568 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 4/1/2006 | GoF | https://clinicaltrials.gov/ct2/show/NCT01515579 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/1/2001 | GoF | https://clinicaltrials.gov/ct2/show/NCT01511159 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.whocc.no/atc_ddd_index/?code=A10BJ02 | http://www.ebi.ac.uk/efo/EFO_0000400 | |||
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00983021 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/1/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT01515553 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 10/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00993720 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| non-alcoholic fatty liver disease | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 8/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02147925 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| major depressive disorder | LIRAGLUTIDE | psychiatric disorder | 0.7 | 3 | Completed | protect | 5/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02423824 | http://purl.obolibrary.org/obo/MONDO_0002009 | |
| morbid obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 4/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02616003 | http://www.ebi.ac.uk/efo/EFO_0001074 | |
| diabetic nephropathy | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01847313 | http://www.ebi.ac.uk/efo/EFO_0000401 | |
| diabetic nephropathy | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 4/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02545738 | http://www.ebi.ac.uk/efo/EFO_0000401 | |
| binge eating | LIRAGLUTIDE | psychiatric disorder | 1 | 4 | Completed | protect | 11/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01739049 | http://www.ebi.ac.uk/efo/EFO_0005924 | |
| Hyperglycemia | LIRAGLUTIDE | phenotype | 0.7 | 3 | Unknown status | protect | 1/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02016846 | http://purl.obolibrary.org/obo/HP_0003074 | |
| insulin resistance | LIRAGLUTIDE | phenotype | 1 | 4 | Unknown status | protect | 3/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02403284 | http://www.ebi.ac.uk/efo/EFO_0002614 | |
| MODY | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 8/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01610934 | http://www.orpha.net/ORDO/Orphanet_552 | |
| pouchitis | LIRAGLUTIDE | gastrointestinal disease | 0.2 | 2 | Recruiting | protect | 3/22/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT04763564 | http://www.ebi.ac.uk/efo/EFO_0003921 | |
| chronic obstructive pulmonary disease | LIRAGLUTIDE | respiratory or thoracic disease | 1 | 4 | Completed | protect | 1/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03466021 | http://www.ebi.ac.uk/efo/EFO_0000341 | |
| sleep apnea | LIRAGLUTIDE | psychiatric disorder | 0.1 | 1 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01832532 | http://www.ebi.ac.uk/efo/EFO_0003877 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Withdrawn | protect | 5/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02109315 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 1/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02408705 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 11/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01722240 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 5/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT02516657 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 11/10/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02443155 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02092896 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 11/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01722266 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Recruiting | protect | 3/21/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05794581 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 5/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02098395 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02777073 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 2/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01782261 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01536665 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02908087 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 1/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01755416 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.05 | 0.5 | Completed | protect | 8/15/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03632759 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Unknown status | protect | 1/1/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03011008 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 8/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02518945 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.05 | 0.5 | Completed | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01856790 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Unknown status | protect | 7/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02584582 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01787916 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 7/1/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05467514 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 11/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02617654 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Enrolling by invitation | protect | 12/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02611232 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 12/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01753362 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Recruiting | protect | 1/1/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03011021 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 11/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01836523 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT01879917 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Active, not recruiting | protect | 8/15/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03182426 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02898506 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| type 1 diabetes mellitus | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 2/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02351232 | http://purl.obolibrary.org/obo/MONDO_0005147 | |
| gestational diabetes | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 7/1/2012 | GoF | https://clinicaltrials.gov/ct2/show/NCT01795248 | http://www.ebi.ac.uk/efo/EFO_0004593 | |
| Parkinson disease | LIRAGLUTIDE | nervous system disease | 0.2 | 2 | Completed | protect | 4/3/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02953665 | http://purl.obolibrary.org/obo/MONDO_0005180 | |
| prediabetes syndrome | LIRAGLUTIDE | endocrine system disease | 1 | 4 | Completed | protect | 5/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02488057 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| prediabetes syndrome | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/1/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT01784965 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| prediabetes syndrome | LIRAGLUTIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01779362 | http://www.ebi.ac.uk/efo/EFO_1001121 | |
| non-alcoholic steatohepatitis | LIRAGLUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/1/2010 | GoF | https://clinicaltrials.gov/ct2/show/NCT01237119 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| Weight loss | LIRAGLUTIDE | phenotype | 0.7 | 3 | Completed | protect | 8/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT04325581 | http://purl.obolibrary.org/obo/HP_0001824 | |
| Malabsorption | LIRAGLUTIDE | phenotype | 1 | 4 | Completed | protect | 3/7/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03955575 | http://purl.obolibrary.org/obo/HP_0002024 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 5/9/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04487743 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/20/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03041792 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 8/18/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02870231 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 8/22/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03341429 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 1/10/2007 | GoF | https://clinicaltrials.gov/ct2/show/NCT00422058 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/26/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03486392 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 1/19/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05579249 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 5/22/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03048578 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03856047 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 9/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02911818 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 6/21/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04883346 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 9/1/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03671733 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Active, not recruiting | protect | 11/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02944500 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 6/22/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03115424 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 6/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01272219 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 8/24/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04311411 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 11/26/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03757130 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 2/6/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02963935 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 9/8/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04575194 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 9/29/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02918279 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 12/18/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02647944 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 11/29/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03523273 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 9/4/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02235961 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 2/6/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT02963922 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Not yet recruiting | protect | 4/1/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04008290 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 3/5/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03347890 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 3/14/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02696148 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 9/11/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04074161 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 6/6/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03500484 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Active, not recruiting | protect | 9/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT04122716 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 2/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01789086 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 6/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01272232 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 9/16/2009 | GoF | https://clinicaltrials.gov/ct2/show/NCT00978393 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 2/10/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT01725126 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 12/11/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03374956 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 3/4/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04775082 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 11/30/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05111912 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 8/18/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04605861 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 11/9/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02527200 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 3/16/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02717858 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 10/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02453711 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 6/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02082496 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 5/24/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04525300 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 10/30/2008 | GoF | https://clinicaltrials.gov/ct2/show/NCT00781937 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 12/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03986008 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | LIRAGLUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 8/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02207348 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| bipolar disorder | LIRAGLUTIDE | psychiatric disorder | 0.7 | 3 | Completed | protect | 5/1/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02423824 | http://purl.obolibrary.org/obo/MONDO_0004985 | |
| metabolic syndrome | LIRAGLUTIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 8/11/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01234649 | http://www.ebi.ac.uk/efo/EFO_0000195 | |
| metabolic syndrome | LIRAGLUTIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 11/1/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04575844 | http://www.ebi.ac.uk/efo/EFO_0000195 | |
| osteoarthritis, knee | LIRAGLUTIDE | musculoskeletal or connective tissue disease | 1 | 4 | Completed | protect | 11/1/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02910570 | http://www.ebi.ac.uk/efo/EFO_0004616 | |
| type 2 diabetes mellitus | EFINOPEGDUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 8/1/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03235219 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| non-alcoholic steatohepatitis | EFINOPEGDUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/4/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04944992 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| obesity | EFINOPEGDUTIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 3/26/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03486392 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | EFINOPEGDUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 6/29/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03586843 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| chronic kidney disease | EFINOPEGDUTIDE | urinary system disease | 0.1 | 1 | Completed | protect | 6/6/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03546205 | http://www.ebi.ac.uk/efo/EFO_0003884 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/7/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03745937 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/2/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03235050 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/8/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03444584 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 1/21/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04208620 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 8/10/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03645421 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 9/27/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04091373 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/31/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03555994 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/9/2015 | GoF | https://clinicaltrials.gov/ct2/show/NCT02548585 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/30/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03341013 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/4/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03244800 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| diabetes mellitus | COTADUTIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 2/25/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05437848 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| Renal insufficiency | COTADUTIDE | phenotype | 0.1 | 1 | Completed | protect | 10/27/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03235375 | http://purl.obolibrary.org/obo/HP_0000083 | |
| non-alcoholic fatty liver disease | COTADUTIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 9/23/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04019561 | http://www.ebi.ac.uk/efo/EFO_0003095 | |
| obesity | COTADUTIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 8/14/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03625778 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 5/7/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03861052 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 3/8/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05706506 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 6/3/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03954834 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 11/30/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05564039 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 5/24/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03131687 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 11/20/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03730662 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 6/28/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03951753 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/9/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04093752 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 8/1/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05433584 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 10/21/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04235959 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 1 | 4 | protect | GoF | https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215866s000lbl.pdf | http://purl.obolibrary.org/obo/MONDO_0005148 | |||
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/1/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03882970 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 10/19/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03311724 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 8/30/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04039503 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 4/13/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05260021 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 11/15/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03322631 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 1 | 4 | Recruiting | protect | 3/15/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05553093 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 5/11/2016 | GoF | https://clinicaltrials.gov/ct2/show/NCT02759107 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 7/30/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03987919 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Active, not recruiting | protect | 5/29/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04255433 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 3/30/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03861039 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 10/19/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04537923 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| diabetes mellitus | TIRZEPATIDE | endocrine system disease | 0.7 | 3 | Recruiting | protect | 2/5/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05691712 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| Wolfram syndrome | TIRZEPATIDE | genetic, familial or congenital disease | 0.2 | 2 | Not yet recruiting | protect | 1/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05659368 | http://purl.obolibrary.org/obo/MONDO_0018105 | |
| non-alcoholic steatohepatitis | TIRZEPATIDE | endocrine system disease | 0.2 | 2 | Active, not recruiting | protect | 11/19/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04166773 | http://www.ebi.ac.uk/efo/EFO_1001249 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.2 | 2 | Recruiting | protect | 2/8/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05536804 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 7/9/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04081337 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/29/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04660643 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.2 | 2 | Not yet recruiting | protect | 6/1/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05912621 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.1 | 1 | Recruiting | protect | 2/7/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05696847 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 10/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05556512 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 12/4/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04184622 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 9/1/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT05024032 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 8/24/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04311411 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Active, not recruiting | protect | 5/10/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04844918 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Completed | protect | 3/29/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04657016 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.7 | 3 | Recruiting | protect | 4/21/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05822830 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | TIRZEPATIDE | nutritional or metabolic disease | 0.1 | 1 | Active, not recruiting | protect | 11/11/2022 | GoF | https://clinicaltrials.gov/ct2/show/NCT05582096 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| chronic kidney disease | TIRZEPATIDE | urinary system disease | 0.2 | 2 | Recruiting | protect | 2/8/2023 | GoF | https://clinicaltrials.gov/ct2/show/NCT05536804 | http://www.ebi.ac.uk/efo/EFO_0003884 | |
| type 2 diabetes mellitus | AVEXITIDE | endocrine system disease | 0.1 | 1 | Completed | protect | 12/1/2006 | LoF | https://clinicaltrials.gov/ct2/show/NCT01449019 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | AVEXITIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 4/15/2021 | LoF | https://clinicaltrials.gov/ct2/show/NCT04466618 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| morbid obesity | AVEXITIDE | nutritional or metabolic disease | 1 | 4 | Recruiting | protect | 4/1/2014 | LoF | https://clinicaltrials.gov/ct2/show/NCT02336659 | http://www.ebi.ac.uk/efo/EFO_0001074 | |
| nutritional disorder | AVEXITIDE | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 11/1/2011 | LoF | https://clinicaltrials.gov/ct2/show/NCT01900340 | http://www.ebi.ac.uk/efo/EFO_0001069 | |
| monogenic diabetes | AVEXITIDE | immune system disease | 0.1 | 1 | Completed | protect | 1/1/2014 | LoF | https://clinicaltrials.gov/ct2/show/NCT01795144 | http://www.ebi.ac.uk/efo/EFO_1001511 | |
| obesity | AVEXITIDE | nutritional or metabolic disease | 1 | 4 | Completed | protect | 1/1/2013 | LoF | https://clinicaltrials.gov/ct2/show/NCT01701973 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | AVEXITIDE | nutritional or metabolic disease | 0.2 | 2 | Withdrawn | protect | 1/1/2016 | LoF | https://clinicaltrials.gov/ct2/show/NCT02697253 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| gastric emptying | AVEXITIDE | biological process | 0.1 | 1 | Completed | protect | 10/1/2004 | LoF | https://clinicaltrials.gov/ct2/show/NCT00980083 | http://purl.obolibrary.org/obo/GO_0035483 | |
| hyperinsulinemic hypoglycemia | AVEXITIDE | endocrine system disease | 0.1 | 1 | Unknown status | protect | 4/1/2015 | LoF | https://clinicaltrials.gov/ct2/show/NCT02996812 | http://www.ebi.ac.uk/efo/EFO_0007318 | |
| hyperinsulinemic hypoglycemia | AVEXITIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 6/21/2021 | LoF | https://clinicaltrials.gov/ct2/show/NCT04652479 | http://www.ebi.ac.uk/efo/EFO_0007318 | |
| Hypoglycemia | AVEXITIDE | phenotype | 0.1 | 1 | Unknown status | protect | 2/1/2014 | LoF | https://clinicaltrials.gov/ct2/show/NCT02550145 | http://purl.obolibrary.org/obo/HP_0001943 | |
| Hypoglycemia | AVEXITIDE | phenotype | 0.2 | 2 | Completed | protect | 5/1/2016 | LoF | https://clinicaltrials.gov/ct2/show/NCT02771574 | http://purl.obolibrary.org/obo/HP_0001943 | |
| Hypoglycemia | AVEXITIDE | phenotype | 0.2 | 2 | Completed | protect | 3/19/2018 | LoF | https://clinicaltrials.gov/ct2/show/NCT03373435 | http://purl.obolibrary.org/obo/HP_0001943 | |
| type 2 diabetes mellitus | DANUGLIPRON | endocrine system disease | 0.2 | 2 | Completed | protect | 10/15/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT03985293 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DANUGLIPRON | endocrine system disease | 0.1 | 1 | Completed | protect | 6/25/2018 | GoF | https://clinicaltrials.gov/ct2/show/NCT03538743 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DANUGLIPRON | endocrine system disease | 0.1 | 1 | Completed | protect | 10/26/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04552470 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | DANUGLIPRON | endocrine system disease | 0.1 | 1 | Completed | protect | 7/7/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04889157 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| liver disease | DANUGLIPRON | endocrine system disease | 0.1 | 1 | Completed | protect | 12/30/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04604496 | http://www.ebi.ac.uk/efo/EFO_0001421 | |
| obesity | DANUGLIPRON | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 12/15/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04621227 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | DANUGLIPRON | nutritional or metabolic disease | 0.2 | 2 | Active, not recruiting | protect | 1/29/2021 | GoF | https://clinicaltrials.gov/ct2/show/NCT04707313 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| obesity | DANUGLIPRON | nutritional or metabolic disease | 0.1 | 1 | Completed | protect | 11/4/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04616339 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02081118 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 1/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02057172 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| type 2 diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.7 | 3 | Completed | protect | 12/5/2017 | GoF | https://clinicaltrials.gov/ct2/show/NCT03353350 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.1 | 1 | Unknown status | protect | 12/1/2013 | GoF | https://clinicaltrials.gov/ct2/show/NCT02059564 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| diabetes mellitus | EFPEGLENATIDE | endocrine system disease | 0.2 | 2 | Completed | protect | 12/1/2011 | GoF | https://clinicaltrials.gov/ct2/show/NCT01452451 | http://www.ebi.ac.uk/efo/EFO_0000400 | |
| obesity | EFPEGLENATIDE | nutritional or metabolic disease | 0.2 | 2 | Completed | protect | 2/1/2014 | GoF | https://clinicaltrials.gov/ct2/show/NCT02075281 | http://www.ebi.ac.uk/efo/EFO_0001073 | |
| type 2 diabetes mellitus | NLY-01 | endocrine system disease | 0.2 | 2 | Completed | protect | 10/16/2019 | GoF | https://clinicaltrials.gov/ct2/show/NCT04159766 | http://purl.obolibrary.org/obo/MONDO_0005148 | |
| Parkinson disease | NLY-01 | nervous system disease | 0.2 | 2 | Active, not recruiting | protect | 2/27/2020 | GoF | https://clinicaltrials.gov/ct2/show/NCT04154072 | http://purl.obolibrary.org/obo/MONDO_0005180 |
In the Platform, ChEMBL evidence represents any target-disease relationship that can be explained by an approved or clinical candidate drug, targeting the gene product and indicated for the disease. Independent studies are treated as individual evidence.
To provide additional context, we integrate a machine learning-based analysis of the reasons why a clinical trial has ended earlier than scheduled. This sorts the stop reasons into a set of 17 classes which include negative, neutral, and positive reasons.