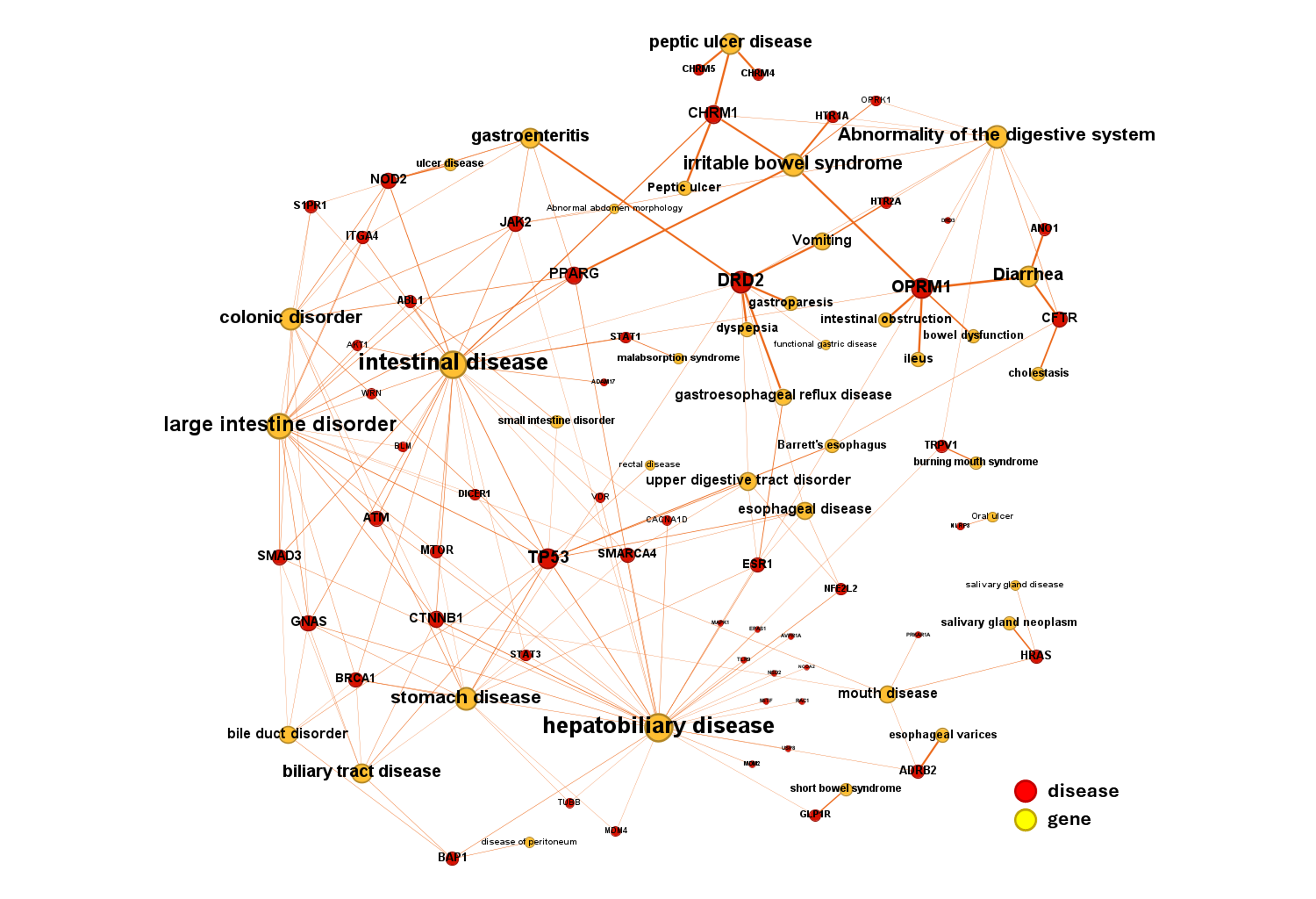
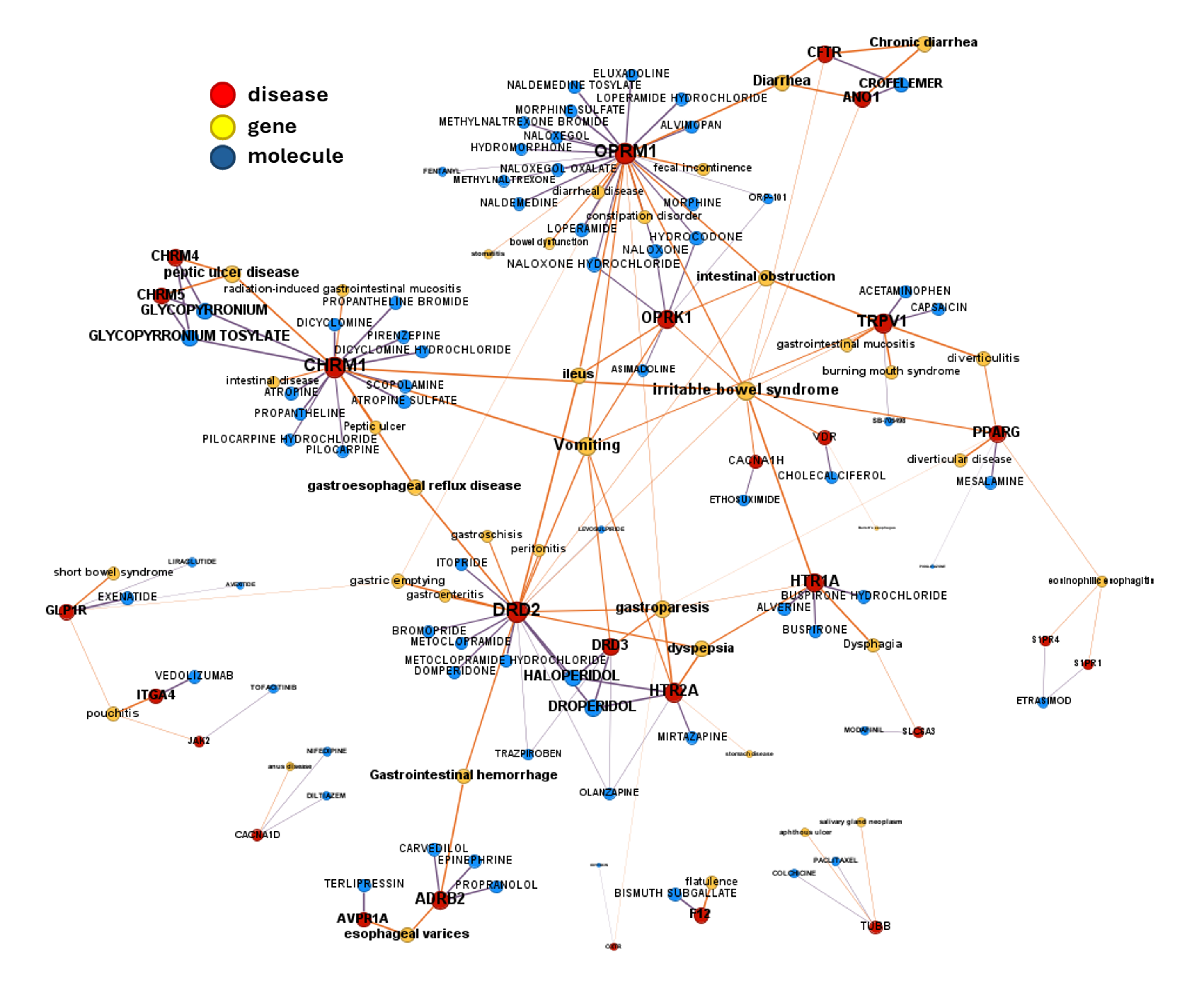
AI-Driven Drug Discovery for Gastrointestinal Diseases
DrTarget harnesses advanced AI and bioinformatics to uncover novel disease-target associations for gastrointestinal (GI) disorders. Using machine learning and pathway analysis, we identify new therapeutic opportunities for:
✔ Inflammatory Bowel Disease (IBD) – Discovering novel targets for Crohn’s disease and ulcerative colitis.
✔ Liver Diseases – Identifying compounds for NAFLD, NASH, and liver fibrosis.
✔ Gastrointestinal Cancers – Enhancing drug discovery for colorectal, gastric, and pancreatic cancers.
✔ Microbiome-Linked Disorders – Exploring AI-based insights into gut microbiota interactions.
Check best scored target-disease associations in table:
| BioAssay Name | program | diseaseName | assayType | associationScore | numberOfEvidences | testedCompounds | activeCompounds |
|---|---|---|---|---|---|---|---|
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Barrett's esophagus | targetBased | 0.235228761404003 | 20 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Barrett's esophagus | targetBased | 0.235228761404003 | 20 | 296501 | 2737 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Barrett's esophagus | pathwayBased | 0.330975192177869 | 416 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Barrett's esophagus | targetBased | 0.330975192177869 | 416 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Barrett's esophagus | targetBased | 0.330975192177869 | 416 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Barrett's esophagus | targetBased | 0.330975192177869 | 416 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Barrett's esophagus | targetBased | 0.330975192177869 | 416 | 124022 | 1156 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | irritable bowel syndrome | targetBased | 0.322758795135049 | 18 | 290355 | 265 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | irritable bowel syndrome | targetBased | 0.419910414935912 | 5 | 394050 | 3624 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | irritable bowel syndrome | targetBased | 0.609584926847712 | 24 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | irritable bowel syndrome | targetBased | 0.609584926847712 | 24 | 335239 | 695 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | irritable bowel syndrome | targetBased | 0.571601682713427 | 23 | 196177 | 519 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | irritable bowel syndrome | targetBased | 0.578284034396309 | 9 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | irritable bowel syndrome | targetBased | 0.578284034396309 | 9 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | irritable bowel syndrome | targetBased | 0.578284034396309 | 9 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | irritable bowel syndrome | targetBased | 0.578284034396309 | 9 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | irritable bowel syndrome | targetBased | 0.578284034396309 | 9 | 359207 | 4555 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | irritable bowel syndrome | targetBased | 0.557687575050289 | 12 | 64908 | 366 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | irritable bowel syndrome | targetBased | 0.557687575050289 | 12 | 61606 | 416 |
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | irritable bowel syndrome | targetBased | 0.304485764646766 | 5 | 104728 | 4230 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | irritable bowel syndrome | targetBased | 0.110654655228409 | 78 | 316642 | 617 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | salivary gland neoplasm | targetBased | 0.466551012569262 | 19 | 194628 | 267 |
| HCS assay for microtubule stabilizers | TUBB | salivary gland neoplasm | targetBased | 0.110381587894355 | 13 | 195821 | 1625 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | necrotizing enterocolitis | targetBased | 0.115710864248473 | 20 | 330392 | 1295 |
| qHTS for Inhibitors of TGF-b | TGFB1 | eosinophilic esophagitis | pathwayBased | 0.104207403721309 | 92 | 403345 | 4970 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | salivary gland neoplasm | pathwayBased | 0.177738024780621 | 69 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland neoplasm | targetBased | 0.177738024780621 | 69 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland neoplasm | targetBased | 0.177738024780621 | 69 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland neoplasm | targetBased | 0.177738024780621 | 69 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland neoplasm | targetBased | 0.177738024780621 | 69 | 124022 | 1156 |
| HTS of Smad transcription factor inhibitors | SMAD3 | eosinophilic esophagitis | targetBased | 0.346515606232344 | 6 | 88033 | 251 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | gastroesophageal reflux disease | targetBased | 0.317249223731667 | 8 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | gastroesophageal reflux disease | targetBased | 0.317249223731667 | 8 | 86095 | 1151 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | gastroesophageal reflux disease | targetBased | 0.611643521081338 | 12 | 336308 | 6862 |
| Assay for HTS of Gi/Go-linked GPCRs using mGluR8: Primary Screening | GRM8 | gastroesophageal reflux disease | targetBased | 0.377619787484508 | 6 | 105151 | 2166 |
| Fluorescence Biochemical Primary HTS to Identify Inhibitors of GASC-1 Activity | KDM4C | gut microbiome measurement | targetBased | 0.145279842104166 | 5 | 326066 | 228 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | dyspepsia | targetBased | 0.614594595559056 | 21 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | dyspepsia | targetBased | 0.614594595559056 | 21 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | dyspepsia | targetBased | 0.614594595559056 | 21 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | dyspepsia | targetBased | 0.614594595559056 | 21 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | dyspepsia | targetBased | 0.614594595559056 | 21 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | dyspepsia | targetBased | 0.614594595559056 | 21 | 336308 | 6862 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | intestinal disease | targetBased | 0.16236887339206 | 186 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | intestinal disease | targetBased | 0.16236887339206 | 186 | 296501 | 2737 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | intestinal disease | targetBased | 0.190247174158658 | 206 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | intestinal disease | targetBased | 0.190247174158658 | 206 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | intestinal disease | targetBased | 0.273474001557521 | 154 | 217959 | 2390 |
| Primary cell-based high throughput screening assay to identify inhibitors of kruppel-like factor 5 (KLF5) | KLF5 | intestinal disease | targetBased | 0.166424068651822 | 38 | 290726 | 671 |
| qHTS for Inhibitors of TGF-b | TGFB1 | intestinal disease | pathwayBased | 0.191051208401801 | 965 | 403345 | 4970 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | intestinal disease | targetBased | 0.183943869471892 | 85 | 57013 | 201 |
| Luminescence-based primary cell-based high throughput screening assay to identify activators of the Aryl Hydrocarbon Receptor (AHR) | AHR_activators | intestinal disease | targetBased | 0.134425221143952 | 356 | 324747 | 7988 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | intestinal disease | pathwayBased | 0.188886131983759 | 1943 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | intestinal disease | pathwayBased | 0.188886131983759 | 1943 | 359244 | 3094 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | intestinal disease | targetBased | 0.22533214842089 | 237 | 394050 | 3624 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | intestinal disease | targetBased | 0.159558530675666 | 1437 | 67275 | 2509 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | intestinal disease | targetBased | 0.223801507523021 | 66 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | intestinal disease | targetBased | 0.223801507523021 | 66 | 335239 | 695 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | intestinal disease | targetBased | 0.165678384527367 | 45 | 405130 | 6428 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | intestinal disease | targetBased | 0.165678384527367 | 45 | 373462 | 23 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | intestinal disease | targetBased | 0.165678384527367 | 45 | 405130 | 6428 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | intestinal disease | pathwayBased | 0.309480347410475 | 434 | 195980 | 5134 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | intestinal disease | targetBased | 0.309480347410475 | 434 | 195980 | 695 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | intestinal disease | targetBased | 0.19875880597046 | 57 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | intestinal disease | targetBased | 0.184464351752167 | 91 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | intestinal disease | pathwayBased | 0.184464351752167 | 91 | 403871 | 1243 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | intestinal disease | targetBased | 0.184367571936416 | 37 | 363803 | 458 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | intestinal disease | targetBased | 0.108088473305537 | 195 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | intestinal disease | targetBased | 0.108088473305537 | 195 | 335180 | 1022 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | intestinal disease | targetBased | 0.285245238122266 | 509 | 196177 | 519 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | intestinal disease | pathwayBased | 0.313419734715544 | 1889 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | intestinal disease | targetBased | 0.313419734715544 | 1889 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | intestinal disease | targetBased | 0.313419734715544 | 1889 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | intestinal disease | targetBased | 0.313419734715544 | 1889 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | intestinal disease | targetBased | 0.313419734715544 | 1889 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | intestinal disease | targetBased | 0.256767340162127 | 593 | 356517 | 1139 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | intestinal disease | targetBased | 0.111457367425371 | 28 | 325630 | 216 |
| Absorbance-based primary biochemical high throughput screening assay to identify activators of procaspase-3 | procaspase3Activators | intestinal disease | targetBased | 0.111468945751082 | 246 | 326024 | 350 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | intestinal disease | targetBased | 0.324110275625142 | 1213 | 292323 | 1836 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | intestinal disease | targetBased | 0.395175882863495 | 12 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | intestinal disease | targetBased | 0.395175882863495 | 12 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | intestinal disease | targetBased | 0.395175882863495 | 12 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | intestinal disease | targetBased | 0.395175882863495 | 12 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | intestinal disease | targetBased | 0.395175882863495 | 12 | 359207 | 4555 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | intestinal disease | pathwayBased | 0.210477469754348 | 774 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | intestinal disease | pathwayBased | 0.210477469754348 | 774 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | intestinal disease | targetBased | 0.172857872797247 | 15 | 339297 | 1446 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | intestinal disease | targetBased | 0.151147465092598 | 43 | 218654 | 8241 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | intestinal disease | targetBased | 0.193425486897253 | 31 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | intestinal disease | targetBased | 0.193425486897253 | 31 | 331360 | 2760 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | intestinal disease | targetBased | 0.19821201084393 | 144 | 316642 | 617 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | intestinal disease | pathwayBased | 0.252632519252451 | 296 | 43989 | 342 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | fecal incontinence | targetBased | 0.543359118242622 | 6 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | fecal incontinence | targetBased | 0.543359118242622 | 6 | 335239 | 695 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | biliary tract disease | targetBased | 0.125954934868726 | 10 | 194152 | 1342 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | biliary tract disease | targetBased | 0.185763128377747 | 68 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | biliary tract disease | targetBased | 0.185763128377747 | 68 | 86095 | 1151 |
| qHTS Assay for the Inhibitors of Human Flap endonuclease 1 (FEN1). | FEN1_inhibitors | biliary tract disease | targetBased | 0.112243454341494 | 7 | 386270 | 1331 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | disease of peritoneum | pathwayBased | 0.181819499029054 | 77 | 376029 | 3978 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | disease of peritoneum | targetBased | 0.160705135248287 | 25 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | disease of peritoneum | targetBased | 0.160705135248287 | 25 | 86095 | 1151 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | disease of peritoneum | pathwayBased | 0.169623621717477 | 108 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disease of peritoneum | targetBased | 0.169623621717477 | 108 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disease of peritoneum | targetBased | 0.169623621717477 | 108 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disease of peritoneum | targetBased | 0.169623621717477 | 108 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disease of peritoneum | targetBased | 0.169623621717477 | 108 | 124022 | 1156 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | esophageal varices | targetBased | 0.57280694202758 | 8 | 339297 | 1446 |
| qHTS of IL-2 Activators | IL2 | malabsorption syndrome | targetBased | 0.165804278457421 | 40 | 364617 | 238 |
| Primary qHTS for Inhibitors of ATXN expression | ATXN2_repressors | malabsorption syndrome | targetBased | 0.15422425171287 | 6 | 358434 | 2554 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | stomach disease | targetBased | 0.148228038380256 | 6 | 336894 | 489 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | stomach disease | targetBased | 0.226248720210366 | 55 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | stomach disease | targetBased | 0.226248720210366 | 55 | 86095 | 1151 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | stomach disease | targetBased | 0.169495776447095 | 14 | 359207 | 1432 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | stomach disease | targetBased | 0.158064897594836 | 16 | 57013 | 201 |
| E3 Ligase HTS_1536 | MDM2 | stomach disease | targetBased | 0.169197340629072 | 29 | 207811 | 220 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | stomach disease | pathwayBased | 0.301248975474893 | 552 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | stomach disease | targetBased | 0.301248975474893 | 552 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | stomach disease | targetBased | 0.301248975474893 | 552 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | stomach disease | targetBased | 0.301248975474893 | 552 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | stomach disease | targetBased | 0.301248975474893 | 552 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | stomach disease | targetBased | 0.18309160563804 | 141 | 356517 | 1139 |
| HTS of Smad transcription factor inhibitors | SMAD3 | stomach disease | targetBased | 0.150959091189651 | 11 | 88033 | 251 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | stomach disease | pathwayBased | 0.177437817873502 | 82 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | stomach disease | pathwayBased | 0.177437817873502 | 82 | 194666 | 1722 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | stomach disease | pathwayBased | 0.200037104961387 | 64 | 43989 | 342 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | gastrointestinal disease | targetBased | 0.338673280198078 | 752 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | gastrointestinal disease | targetBased | 0.338673280198078 | 752 | 296501 | 2737 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | gastrointestinal disease | targetBased | 0.549751654009714 | 39 | 290355 | 265 |
| qHTS assay for MDR1-selective chemotherapeutics: Primary screen using the drug-selected MDR subline cells KB-V1 | MDR1-selective compounds | gastrointestinal disease | targetBased | 0.103336312144793 | 479 | 396029 | 13426 |
| qHTS assay for MDR1-selective chemotherapeutics: Primary screen using the parental adenocarcinoma cell KB-3-1 | MDR1-selective compounds | gastrointestinal disease | targetBased | 0.103336312144793 | 479 | 395981 | 5516 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | gastrointestinal disease | targetBased | 0.298709473653827 | 203 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | gastrointestinal disease | targetBased | 0.298709473653827 | 203 | 337446 | 1356 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | gastrointestinal disease | targetBased | 0.280710885313529 | 1047 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | gastrointestinal disease | targetBased | 0.280710885313529 | 1047 | 86095 | 1151 |
| qHTS for Inhibitors of TGF-b | TGFB1 | gastrointestinal disease | pathwayBased | 0.218983632603833 | 5317 | 403345 | 4970 |
| Luminescence-based primary cell-based high throughput screening assay to identify activators of the Aryl Hydrocarbon Receptor (AHR) | AHR_activators | gastrointestinal disease | targetBased | 0.138082050917971 | 782 | 324747 | 7988 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | gastrointestinal disease | pathwayBased | 0.203873417453037 | 5969 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | gastrointestinal disease | pathwayBased | 0.203873417453037 | 5969 | 359244 | 3094 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | gastrointestinal disease | targetBased | 0.254736303304555 | 874 | 394050 | 3624 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | gastrointestinal disease | targetBased | 0.159294284204697 | 4482 | 67275 | 2509 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | gastrointestinal disease | targetBased | 0.626780891642088 | 146 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | gastrointestinal disease | targetBased | 0.626780891642088 | 146 | 335239 | 695 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | gastrointestinal disease | targetBased | 0.278303064132598 | 4612 | 405130 | 6428 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | gastrointestinal disease | targetBased | 0.278303064132598 | 4612 | 373462 | 23 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | gastrointestinal disease | targetBased | 0.278303064132598 | 4612 | 405130 | 6428 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | gastrointestinal disease | pathwayBased | 0.311829263265792 | 836 | 195980 | 5134 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | gastrointestinal disease | targetBased | 0.311829263265792 | 836 | 195980 | 695 |
| qHTS for Inhibitors of Inflammasome Signaling: IL-1-beta AlphaLISA Primary Screen | IL-1b Inflammasome | gastrointestinal disease | pathwayBased | 0.151421656658236 | 5514 | 362051 | 17187 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastrointestinal disease | targetBased | 0.375465575819017 | 3259 | 196177 | 519 |
| VEID(2) R110 Enzymatic Primary HTS to identify Inhibitors of Caspase 6 Measured in Biochemical System Using Plate Reader - 7052-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | Caspase6Inhibitors | gastrointestinal disease | targetBased | 0.12979173562019 | 37 | 344318 | 1854 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | gastrointestinal disease | pathwayBased | 0.329006687360773 | 8564 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastrointestinal disease | targetBased | 0.329006687360773 | 8564 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastrointestinal disease | targetBased | 0.329006687360773 | 8564 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastrointestinal disease | targetBased | 0.329006687360773 | 8564 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastrointestinal disease | targetBased | 0.329006687360773 | 8564 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | gastrointestinal disease | targetBased | 0.26636181111914 | 5746 | 356517 | 1139 |
| Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer (HTRF) Assay | CACNA1B_modulators | gastrointestinal disease | targetBased | 0.406070807924668 | 7 | 292323 | 567 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | gastrointestinal disease | targetBased | 0.630154237489178 | 108 | 336308 | 6862 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | gastrointestinal disease | targetBased | 0.552312264766224 | 68 | 335531 | 328 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | gastrointestinal disease | targetBased | 0.118840680082599 | 2239 | 330392 | 1295 |
| uHTS Luminescent assay for identification of inhibitors of human intestinal alkaline phosphatase | ALPI_inhibitors | gastrointestinal disease | targetBased | 0.19138442576141 | 40 | 330392 | 393 |
| uHTS Luminescent assay for identification of inhibitors of mouse intestinal alkaline phosphatase | ALPI_inhibitors | gastrointestinal disease | targetBased | 0.19138442576141 | 40 | 331670 | 664 |
| Luminescent assay for identification of activators of bovine intestinal alkaline phosphatase | ALPI_activators | gastrointestinal disease | targetBased | 0.19138442576141 | 40 | 195570 | 326 |
| uHTS Luminescent assay for identification of activators of human intestinal alkaline phosphatase | ALPI_activators | gastrointestinal disease | targetBased | 0.19138442576141 | 40 | 330392 | 537 |
| uHTS Luminescent assay for identification of activators of mouse intestinal alkaline phosphatase | ALPI_activators | gastrointestinal disease | targetBased | 0.19138442576141 | 40 | 331670 | 785 |
| Absorbance-based primary biochemical high throughput screening assay to identify activators of procaspase-3 | procaspase3Activators | gastrointestinal disease | targetBased | 0.149094352026461 | 1461 | 326024 | 350 |
| Modulation of AMPAR-stargazin complexes | AMPAStargazinComplexModulators | gastrointestinal disease | targetBased | 0.422744545189202 | 4 | 40473 | 1400 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | gastrointestinal disease | targetBased | 0.333536313063467 | 1311 | 292323 | 1836 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | gastrointestinal disease | targetBased | 0.647353283120319 | 44 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | gastrointestinal disease | targetBased | 0.647353283120319 | 44 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | gastrointestinal disease | targetBased | 0.647353283120319 | 44 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | gastrointestinal disease | targetBased | 0.647353283120319 | 44 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | gastrointestinal disease | targetBased | 0.647353283120319 | 44 | 359207 | 4555 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | gastrointestinal disease | pathwayBased | 0.274561257666288 | 2850 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | gastrointestinal disease | pathwayBased | 0.274561257666288 | 2850 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | gastrointestinal disease | targetBased | 0.230047515561731 | 118 | 339297 | 1446 |
| Primary biochemical high throughput screening assay to identify inhibitors of BCL2-related protein, long isoform (BCLXL). | BCL2L1_modulators | gastrointestinal disease | targetBased | 0.124423196455013 | 398 | 314998 | 2199 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn6) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | gastrointestinal disease | targetBased | 0.232995771004277 | 35 | 369953 | 760 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn7) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | gastrointestinal disease | targetBased | 0.232995771004277 | 35 | 369953 | 498 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | gastrointestinal disease | targetBased | 0.284832737223483 | 30 | 64908 | 366 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | gastrointestinal disease | targetBased | 0.284832737223483 | 30 | 61606 | 416 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | gastrointestinal disease | targetBased | 0.492691737550043 | 9 | 363803 | 1450 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | gastrointestinal disease | targetBased | 0.492691737550043 | 9 | 363803 | 2629 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | gastrointestinal disease | targetBased | 0.492691737550043 | 9 | 363803 | 502 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | gastrointestinal disease | targetBased | 0.490885862309666 | 7 | 363803 | 698 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | gastrointestinal disease | targetBased | 0.490885862309666 | 7 | 363803 | 2133 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | gastrointestinal disease | targetBased | 0.490885862309666 | 7 | 363803 | 1081 |
| HCS assay for microtubule stabilizers | TUBB | gastrointestinal disease | targetBased | 0.285951021888019 | 730 | 195821 | 1625 |
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | gastrointestinal disease | targetBased | 0.449925211715772 | 23 | 104728 | 4230 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | gastrointestinal disease | targetBased | 0.286693450126032 | 377 | 316642 | 617 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | hepatobiliary disease | targetBased | 0.182876083519353 | 82 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | hepatobiliary disease | targetBased | 0.182876083519353 | 82 | 296501 | 2737 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | hepatobiliary disease | targetBased | 0.278278862478759 | 668 | 196177 | 519 |
| E3 Ligase HTS_1536 | MDM2 | hepatobiliary disease | targetBased | 0.226144721997156 | 175 | 207811 | 220 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | hepatobiliary disease | pathwayBased | 0.31053149872031 | 3174 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | hepatobiliary disease | targetBased | 0.31053149872031 | 3174 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | hepatobiliary disease | targetBased | 0.31053149872031 | 3174 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | hepatobiliary disease | targetBased | 0.31053149872031 | 3174 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | hepatobiliary disease | targetBased | 0.31053149872031 | 3174 | 124022 | 1156 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Salivary Gland Pleomorphic Adenoma | pathwayBased | 0.306057893011864 | 5 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Salivary Gland Pleomorphic Adenoma | targetBased | 0.306057893011864 | 5 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Salivary Gland Pleomorphic Adenoma | targetBased | 0.306057893011864 | 5 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Salivary Gland Pleomorphic Adenoma | targetBased | 0.306057893011864 | 5 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Salivary Gland Pleomorphic Adenoma | targetBased | 0.306057893011864 | 5 | 124022 | 1156 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | Salivary Gland Pleomorphic Adenoma | targetBased | 0.407840526197416 | 5 | 193542 | 587 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Colon Dysplasia | pathwayBased | 0.203615980189334 | 5 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Colon Dysplasia | targetBased | 0.203615980189334 | 5 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Colon Dysplasia | targetBased | 0.203615980189334 | 5 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Colon Dysplasia | targetBased | 0.203615980189334 | 5 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Colon Dysplasia | targetBased | 0.203615980189334 | 5 | 124022 | 1156 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | burning mouth syndrome | targetBased | 0.409331474917967 | 23 | 316642 | 617 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | gastroparesis | targetBased | 0.609010949181426 | 33 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | gastroparesis | targetBased | 0.609010949181426 | 33 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | gastroparesis | targetBased | 0.609010949181426 | 33 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | gastroparesis | targetBased | 0.609010949181426 | 33 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | gastroparesis | targetBased | 0.609010949181426 | 33 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | gastroparesis | targetBased | 0.609010949181426 | 33 | 336308 | 6862 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | mouth disease | targetBased | 0.145216007258082 | 54 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | mouth disease | targetBased | 0.145216007258082 | 54 | 86095 | 1151 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | mouth disease | targetBased | 0.189030769016559 | 89 | 394050 | 3624 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | mouth disease | pathwayBased | 0.22524520621551 | 643 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth disease | targetBased | 0.22524520621551 | 643 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth disease | targetBased | 0.22524520621551 | 643 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth disease | targetBased | 0.22524520621551 | 643 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth disease | targetBased | 0.22524520621551 | 643 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | mouth disease | targetBased | 0.1449037414548 | 359 | 356517 | 1139 |
| HTS of Smad transcription factor inhibitors | SMAD3 | mouth disease | targetBased | 0.13858440843348 | 14 | 88033 | 251 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | gastroenteritis | targetBased | 0.108998147043946 | 81 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | gastroenteritis | targetBased | 0.108998147043946 | 81 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | gastroenteritis | targetBased | 0.255744328287688 | 76 | 217959 | 2390 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | gastroenteritis | targetBased | 0.110274934508659 | 28 | 57013 | 201 |
| Luminescence-based primary cell-based high throughput screening assay to identify activators of the Aryl Hydrocarbon Receptor (AHR) | AHR_activators | gastroenteritis | targetBased | 0.124113249622636 | 207 | 324747 | 7988 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | gastroenteritis | pathwayBased | 0.178821024895133 | 1089 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | gastroenteritis | pathwayBased | 0.178821024895133 | 1089 | 359244 | 3094 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | gastroenteritis | targetBased | 0.155693653512334 | 774 | 67275 | 2509 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | gastroenteritis | targetBased | 0.144951058771754 | 13 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | gastroenteritis | targetBased | 0.144951058771754 | 13 | 335239 | 695 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | gastroenteritis | targetBased | 0.236302572320261 | 274 | 196177 | 519 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | gastroenteritis | pathwayBased | 0.113098173508316 | 236 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastroenteritis | targetBased | 0.113098173508316 | 236 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastroenteritis | targetBased | 0.113098173508316 | 236 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastroenteritis | targetBased | 0.113098173508316 | 236 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | gastroenteritis | targetBased | 0.113098173508316 | 236 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | gastroenteritis | targetBased | 0.112720450499552 | 198 | 356517 | 1139 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | gastroenteritis | pathwayBased | 0.138608902911651 | 33 | 7671 | 874 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | gastroenteritis | targetBased | 0.138608902911651 | 33 | 304060 | 10600 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | gastroenteritis | pathwayBased | 0.138608902911651 | 33 | 305439 | 16717 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | gastroenteritis | targetBased | 0.562663370717272 | 11 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | gastroenteritis | targetBased | 0.562663370717272 | 11 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | gastroenteritis | targetBased | 0.562663370717272 | 11 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | gastroenteritis | targetBased | 0.562663370717272 | 11 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | gastroenteritis | targetBased | 0.562663370717272 | 11 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | gastroenteritis | targetBased | 0.562663370717272 | 11 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | gastroenteritis | targetBased | 0.106680740940599 | 16 | 322361 | 619 |
| MLPCN ERAP1 Measured in Biochemical System Using Plate Reader - 7016-01_Inhibitor_SinglePoint_HTS_Activity | ERAP1_inhibitors | gastroenteritis | targetBased | 0.119241205060252 | 4 | 335777 | 499 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | gastroenteritis | targetBased | 0.274271028756649 | 220 | 292323 | 1836 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | gastroenteritis | pathwayBased | 0.124240109384012 | 366 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | gastroenteritis | pathwayBased | 0.124240109384012 | 366 | 194666 | 1722 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | gastroenteritis | pathwayBased | 0.143803635544244 | 95 | 43989 | 342 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | gastroenteritis | targetBased | 0.120926352110174 | 74 | 343468 | 734 |
| uHTS Luminescent assay for identification of inhibitors of NALP3 in yeast | NLRP3 | Oral ulcer | targetBased | 0.259137631040282 | 8 | 330392 | 1295 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | Vomiting | targetBased | 0.442111797582819 | 8 | 363803 | 2412 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | Vomiting | targetBased | 0.607745142219739 | 20 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | Vomiting | targetBased | 0.607745142219739 | 20 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | Vomiting | targetBased | 0.607745142219739 | 20 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | Vomiting | targetBased | 0.607745142219739 | 20 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | Vomiting | targetBased | 0.607745142219739 | 20 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | Vomiting | targetBased | 0.607745142219739 | 20 | 336308 | 6862 |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | Vomiting | targetBased | 0.439677534307829 | 6 | 364051 | 9106 |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | Vomiting | targetBased | 0.439677534307829 | 6 | 407539 | 2380 |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | Vomiting | targetBased | 0.439677534307829 | 6 | 407539 | 2380 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Diarrhea | targetBased | 0.594015846742141 | 46 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Diarrhea | targetBased | 0.594015846742141 | 46 | 296501 | 2737 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Diarrhea | targetBased | 0.664483849517651 | 134 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Diarrhea | targetBased | 0.664483849517651 | 134 | 335239 | 695 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | Diarrhea | targetBased | 0.587578994481585 | 15 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | Diarrhea | targetBased | 0.587578994481585 | 15 | 335180 | 1022 |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | Dysphagia | targetBased | 0.141863454192519 | 5 | 108286 | 1415 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | Gastrointestinal hemorrhage | targetBased | 0.485319970713906 | 5 | 336308 | 6862 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | Peptic ulcer | targetBased | 0.624294296119937 | 14 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | Peptic ulcer | targetBased | 0.624294296119937 | 14 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | Peptic ulcer | targetBased | 0.624294296119937 | 14 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | Peptic ulcer | targetBased | 0.624294296119937 | 14 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | Peptic ulcer | targetBased | 0.624294296119937 | 14 | 359207 | 4555 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the gastrointestinal tract | targetBased | 0.175508109727307 | 14 | 196177 | 519 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | cholestasis | targetBased | 0.512356311300188 | 11 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | cholestasis | targetBased | 0.512356311300188 | 11 | 296501 | 2737 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | cholestasis | targetBased | 0.256611920238613 | 5 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | cholestasis | targetBased | 0.256611920238613 | 5 | 337446 | 1356 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | constipation disorder | targetBased | 0.577168330395272 | 6 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | constipation disorder | targetBased | 0.577168330395272 | 6 | 335239 | 695 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | esophagitis | pathwayBased | 0.204041187380841 | 4 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophagitis | targetBased | 0.204041187380841 | 4 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophagitis | targetBased | 0.204041187380841 | 4 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophagitis | targetBased | 0.204041187380841 | 4 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophagitis | targetBased | 0.204041187380841 | 4 | 124022 | 1156 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | bile duct disorder | targetBased | 0.17334226668571 | 38 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | bile duct disorder | targetBased | 0.17334226668571 | 38 | 86095 | 1151 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | bile duct disorder | pathwayBased | 0.118169891203577 | 76 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | bile duct disorder | pathwayBased | 0.118169891203577 | 76 | 359244 | 3094 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | diverticulitis | targetBased | 0.375440031217794 | 6 | 196177 | 519 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | peptic ulcer disease | targetBased | 0.619285523949174 | 12 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | peptic ulcer disease | targetBased | 0.619285523949174 | 12 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | peptic ulcer disease | targetBased | 0.619285523949174 | 12 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | peptic ulcer disease | targetBased | 0.619285523949174 | 12 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | peptic ulcer disease | targetBased | 0.619285523949174 | 12 | 359207 | 4555 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 1450 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 2629 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 502 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 698 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 2133 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | peptic ulcer disease | targetBased | 0.60466648961276 | 9 | 363803 | 1081 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | bowel dysfunction | targetBased | 0.479330316738609 | 19 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | bowel dysfunction | targetBased | 0.479330316738609 | 19 | 335239 | 695 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | colonic disorder | targetBased | 0.131520063534352 | 33 | 336894 | 489 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | colonic disorder | targetBased | 0.171854369131705 | 109 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | colonic disorder | targetBased | 0.171854369131705 | 109 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | colonic disorder | targetBased | 0.263358302353305 | 84 | 217959 | 2390 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | colonic disorder | pathwayBased | 0.166154154319815 | 1129 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | colonic disorder | pathwayBased | 0.166154154319815 | 1129 | 359244 | 3094 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | colonic disorder | targetBased | 0.151048916654347 | 822 | 67275 | 2509 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | colonic disorder | targetBased | 0.136528383743459 | 58 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | colonic disorder | pathwayBased | 0.136528383743459 | 58 | 403871 | 1243 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | colonic disorder | targetBased | 0.282976593337145 | 320 | 196177 | 519 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | colonic disorder | targetBased | 0.104157290114041 | 52 | 86095 | 1114 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | colonic disorder | targetBased | 0.188079542433228 | 292 | 356517 | 1139 |
| HTS of Smad transcription factor inhibitors | SMAD3 | colonic disorder | targetBased | 0.234442030805364 | 43 | 88033 | 251 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | colonic disorder | targetBased | 0.276202125406148 | 222 | 292323 | 1836 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | colonic disorder | pathwayBased | 0.198003595684103 | 127 | 43989 | 342 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | intestinal obstruction | targetBased | 0.411120019776223 | 6 | 290355 | 265 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | intestinal obstruction | targetBased | 0.634364034199603 | 37 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | intestinal obstruction | targetBased | 0.634364034199603 | 37 | 335239 | 695 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | intestinal obstruction | targetBased | 0.406696647852918 | 5 | 316642 | 617 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | ileus | targetBased | 0.426198928396305 | 5 | 290355 | 265 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | ileus | targetBased | 0.624516078630663 | 44 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | ileus | targetBased | 0.624516078630663 | 44 | 335239 | 695 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | short bowel syndrome | targetBased | 0.427965459731281 | 9 | 405130 | 6428 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | short bowel syndrome | targetBased | 0.427965459731281 | 9 | 373462 | 23 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | short bowel syndrome | targetBased | 0.427965459731281 | 9 | 405130 | 6428 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | small intestine disorder | pathwayBased | 0.201464271300111 | 92 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | small intestine disorder | targetBased | 0.201464271300111 | 92 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | small intestine disorder | targetBased | 0.201464271300111 | 92 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | small intestine disorder | targetBased | 0.201464271300111 | 92 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | small intestine disorder | targetBased | 0.201464271300111 | 92 | 124022 | 1156 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | small intestine disorder | targetBased | 0.137631854483729 | 5 | 316642 | 617 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | enteritis | targetBased | 0.101338531490936 | 17 | 292323 | 1836 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | ulcer disease | targetBased | 0.401332681215866 | 9 | 292323 | 1836 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | salivary gland neoplasm | pathwayBased | 0.139823724490481 | 6 | 376029 | 3978 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | salivary gland neoplasm | targetBased | 0.127450360507124 | 5 | 57013 | 201 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | salivary gland neoplasm | targetBased | 0.102240315993491 | 9 | 356517 | 1139 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | salivary gland neoplasm | targetBased | 0.19671703072317 | 11 | 193542 | 587 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | salivary gland neoplasm | pathwayBased | 0.131101168001345 | 11 | 43989 | 342 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | parotid disease | pathwayBased | 0.151426468445126 | 9 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | parotid disease | targetBased | 0.151426468445126 | 9 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | parotid disease | targetBased | 0.151426468445126 | 9 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | parotid disease | targetBased | 0.151426468445126 | 9 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | parotid disease | targetBased | 0.151426468445126 | 9 | 124022 | 1156 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | salivary gland disease | pathwayBased | 0.139823724490481 | 6 | 376029 | 3978 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | salivary gland disease | targetBased | 0.127495764920373 | 6 | 57013 | 201 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | salivary gland disease | pathwayBased | 0.166045628587948 | 64 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland disease | targetBased | 0.166045628587948 | 64 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland disease | targetBased | 0.166045628587948 | 64 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland disease | targetBased | 0.166045628587948 | 64 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | salivary gland disease | targetBased | 0.166045628587948 | 64 | 124022 | 1156 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | salivary gland disease | targetBased | 0.144855569468029 | 4 | 322361 | 619 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | salivary gland disease | targetBased | 0.19671703072317 | 11 | 193542 | 587 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | salivary gland disease | targetBased | 0.223124685070182 | 18 | 194628 | 267 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | salivary gland disease | pathwayBased | 0.131156363396237 | 13 | 43989 | 342 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | salivary gland disease | targetBased | 0.183738498677122 | 8 | 343468 | 734 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | salivary gland disease | targetBased | 0.102481516064321 | 12 | 356517 | 1139 |
| HTS for developing T Cell Immune Modulators | ITGAL | intestinal disease | targetBased | 0.101430783306978 | 16 | 326271 | 221 |
| Fluorescence-based primary biochemical high throughput screening assay to identify inhibitors of Protein Phosphatase 5 (PP5). | PPP5C | intestinal disease | targetBased | 0.12881045535508 | 4 | 314999 | 564 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | intestinal disease | pathwayBased | 0.236565471108405 | 186 | 376029 | 3978 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | intestinal disease | pathwayBased | 0.102508019793238 | 24 | 64908 | 979 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | intestinal disease | pathwayBased | 0.102508019793238 | 24 | 64908 | 278 |
| uHTS fluorescence polarization assay for the identification of translation initiation inhibitors (PABP) | PABPC1 | intestinal disease | targetBased | 0.10463613014089 | 11 | 290915 | 2982 |
| uHTS identification of microRNA-mediated mRNA deadenylation inhibitors by fluoresence polarization assay | PABPC1 | intestinal disease | targetBased | 0.10463613014089 | 11 | 359244 | 1307 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | intestinal disease | pathwayBased | 0.151214947453312 | 9 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | intestinal disease | targetBased | 0.159574796533532 | 53 | 336894 | 489 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | intestinal disease | targetBased | 0.121122860285039 | 35 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | intestinal disease | targetBased | 0.121122860285039 | 35 | 290726 | 2649 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | intestinal disease | targetBased | 0.179394964517329 | 14 | 290355 | 265 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 1 (SRC1; NCOA1) | NCOA1 | intestinal disease | targetBased | 0.102666967902203 | 6 | 359206 | 428 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | intestinal disease | targetBased | 0.267351541529491 | 48 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | intestinal disease | targetBased | 0.267351541529491 | 48 | 337446 | 1356 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | intestinal disease | targetBased | 0.153711129775858 | 6 | 385746 | 932 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | intestinal disease | targetBased | 0.159191597687086 | 10 | 194152 | 1342 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | intestinal disease | targetBased | 0.261152368093564 | 182 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | intestinal disease | pathwayBased | 0.170037717925935 | 41 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | intestinal disease | pathwayBased | 0.170037717925935 | 41 | 131324 | 544 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | intestinal disease | targetBased | 0.139541898132279 | 11 | 143816 | 859 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | intestinal disease | targetBased | 0.207537686340734 | 29 | 46715 | 2829 |
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | intestinal disease | targetBased | 0.185608790570996 | 8 | 564288 | 6030 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | intestinal disease | targetBased | 0.159367964020923 | 7 | 343468 | 285 |
| qHTS of IL-2 Activators | IL2 | intestinal disease | targetBased | 0.120149762342543 | 420 | 364617 | 238 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | intestinal disease | targetBased | 0.188741361625401 | 7 | 309684 | 1662 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | intestinal disease | targetBased | 0.113191759955279 | 51 | 338328 | 2501 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | intestinal disease | targetBased | 0.263136553708477 | 80 | 326888 | 645 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | intestinal disease | targetBased | 0.1341553957767 | 36 | 75924 | 1804 |
| qHTS for Inhibitors of Cell Surface uPA Generation | PLAU | intestinal disease | targetBased | 0.15565362846769 | 45 | 325247 | 1021 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | intestinal disease | targetBased | 0.193550533035486 | 16 | 369953 | 3123 |
| HTS for PAX8 inhibitors using PAX8 luciferase reporter gene assay in RMG-I cells Measured in Cell-Based System Using Plate Reader - 7054-01_Inhibitor_SinglePoint_HTS_Activity | PAX8 | intestinal disease | targetBased | 0.104982309329976 | 20 | 353950 | 4145 |
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | intestinal disease | targetBased | 0.198625686524713 | 23 | 217959 | 771 |
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | intestinal disease | targetBased | 0.198625686524713 | 23 | 217959 | 569 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | intestinal disease | targetBased | 0.185376160671588 | 6 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | intestinal disease | targetBased | 0.21027293181987 | 34 | 344733 | 7043 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | intestinal disease | targetBased | 0.154533719790588 | 8 | 47480 | 2010 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 2 (SRC2; NCOA2) | NCOA2 | intestinal disease | targetBased | 0.155482904136805 | 6 | 368927 | 620 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | intestinal disease | pathwayBased | 0.147071801746032 | 59 | 7671 | 874 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | intestinal disease | targetBased | 0.147071801746032 | 59 | 304060 | 10600 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | intestinal disease | pathwayBased | 0.147071801746032 | 59 | 305439 | 16717 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | intestinal disease | targetBased | 0.205957757111476 | 16 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | intestinal disease | targetBased | 0.205957757111476 | 16 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | intestinal disease | targetBased | 0.205957757111476 | 16 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | intestinal disease | targetBased | 0.205957757111476 | 16 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | intestinal disease | targetBased | 0.205957757111476 | 16 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | intestinal disease | targetBased | 0.205957757111476 | 16 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | intestinal disease | targetBased | 0.287176355151255 | 100 | 322361 | 619 |
| QFRET-based biochemical primary high throughput screening assay to identify exosite inhibitors of ADAM17. | ADAM17_inhibitors | intestinal disease | targetBased | 0.262685841878716 | 273 | 369953 | 3080 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | intestinal disease | targetBased | 0.196755245992988 | 43 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | intestinal disease | targetBased | 0.168947125242561 | 70 | 207811 | 220 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | RAC1 | intestinal disease | targetBased | 0.162299840619863 | 8 | 194629 | 219 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | RAC1 | intestinal disease | targetBased | 0.162299840619863 | 8 | 194628 | 521 |
| High throughput screening of activators of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | intestinal disease | targetBased | 0.149041588345448 | 6 | 305610 | 382 |
| High throughput screening of inhibitors of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | intestinal disease | targetBased | 0.149041588345448 | 6 | 305610 | 3253 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | intestinal disease | targetBased | 0.200559073805117 | 11 | 335531 | 328 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | intestinal disease | targetBased | 0.157213438775602 | 20 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | intestinal disease | targetBased | 0.157213438775602 | 20 | 218234 | 1620 |
| uHTS Luminescent assay for identification of inhibitors of human intestinal alkaline phosphatase | ALPI_inhibitors | intestinal disease | targetBased | 0.184459425324749 | 22 | 330392 | 393 |
| uHTS Luminescent assay for identification of inhibitors of mouse intestinal alkaline phosphatase | ALPI_inhibitors | intestinal disease | targetBased | 0.184459425324749 | 22 | 331670 | 664 |
| Luminescent assay for identification of activators of bovine intestinal alkaline phosphatase | ALPI_activators | intestinal disease | targetBased | 0.184459425324749 | 22 | 195570 | 326 |
| uHTS Luminescent assay for identification of activators of human intestinal alkaline phosphatase | ALPI_activators | intestinal disease | targetBased | 0.184459425324749 | 22 | 330392 | 537 |
| uHTS Luminescent assay for identification of activators of mouse intestinal alkaline phosphatase | ALPI_activators | intestinal disease | targetBased | 0.184459425324749 | 22 | 331670 | 785 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | intestinal disease | targetBased | 0.166010622733042 | 18 | 71016 | 346 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | intestinal disease | targetBased | 0.156949732612937 | 37 | 148480 | 6428 |
| MLPCN ERAP1 Measured in Biochemical System Using Plate Reader - 7016-01_Inhibitor_SinglePoint_HTS_Activity | ERAP1_inhibitors | intestinal disease | targetBased | 0.144737410208928 | 12 | 335777 | 499 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of HIV-1 LEDGF/p75 DNA Integration | PSIP1 | intestinal disease | targetBased | 0.194833126217206 | 31 | 369953 | 2353 |
| qHTS for Inhibitors of WRN Helicase | WRN | intestinal disease | targetBased | 0.24895238630978 | 17 | 364011 | 1678 |
| Antagonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | intestinal disease | targetBased | 0.145316836034764 | 8 | 329153 | 670 |
| Inverse Agonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | intestinal disease | targetBased | 0.145316836034764 | 8 | 329153 | 670 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor | TSHR | intestinal disease | targetBased | 0.145316836034764 | 8 | 72026 | 352 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor: Activators of Intracellular cAMP Concentrations in Parental HEK 293 | TSHR | intestinal disease | targetBased | 0.145316836034764 | 8 | 72026 | 1794 |
| HTS of Smad transcription factor inhibitors | SMAD3 | intestinal disease | targetBased | 0.281779797644813 | 96 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | intestinal disease | targetBased | 0.268586025959039 | 367 | 193542 | 587 |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of human platelet-activating factor acetylhydrolase 1b, catalytic subunit 2 (PAFAH1B2) | PAFAH1B2 | intestinal disease | targetBased | 0.105013711742327 | 5 | 335239 | 4158 |
| qHTS Assay for the Inhibitors of Human Flap endonuclease 1 (FEN1). | FEN1_inhibitors | intestinal disease | targetBased | 0.104398377572245 | 12 | 386270 | 1331 |
| OTUD3 deubiquitinase inhibition: Primary qHTS | OTUD3 | intestinal disease | targetBased | 0.117665144667545 | 23 | 47480 | 334 |
| Inhibition of the MLL-AF4-AF9 Interaction in Pediatric Leukemia Measured in Biochemical System Using Plate Reader - 2160-01_Inhibitor_SinglePoint_HTS_Activity | MLLT3 | intestinal disease | targetBased | 0.102347892930117 | 4 | 344459 | 1627 |
| SMM ID4 Measured in Biochemical System Using Small Molecule MicroArray - 2128-01_Other_SinglePoint_HTS_Activity | ID4 | intestinal disease | targetBased | 0.109491079300376 | 5 | 31324 | 362 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | intestinal disease | targetBased | 0.19635482496637 | 58 | 194628 | 267 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | intestinal disease | targetBased | 0.196021147190473 | 9 | 64908 | 366 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | intestinal disease | targetBased | 0.196021147190473 | 9 | 61606 | 416 |
| HCS assay for microtubule stabilizers | TUBB | intestinal disease | targetBased | 0.138762726678409 | 27 | 195821 | 1625 |
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | intestinal disease | targetBased | 0.101567813208114 | 4 | 104728 | 4230 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | intestinal disease | targetBased | 0.208105293538328 | 14 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | intestinal disease | targetBased | 0.181342263492155 | 11 | 331671 | 10022 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | intestinal disease | targetBased | 0.12753951187052 | 141 | 343468 | 734 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of the interaction of nucleotide-binding oligomerization domain containing 2 (NOD2) and the receptor-interacting serine-threonine kinase 2 (RIPK2) | RIPK2 | intestinal disease | targetBased | 0.134030120785673 | 38 | 363803 | 1383 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | intestinal disease | targetBased | 0.108033246220152 | 90 | 86095 | 1114 |
| uHTS of small molecular inhibitors for p47phox, a regulatory protein of NADPH oxidases (Noxs) | p47phoxInhibitors | intestinal disease | targetBased | 0.104246077782217 | 19 | 217454 | 1142 |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | intestinal disease | targetBased | 0.215729122095815 | 81 | 55710 | 315 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | biliary tract disease | targetBased | 0.174954934381303 | 35 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | biliary tract disease | targetBased | 0.174954934381303 | 35 | 296501 | 2737 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | biliary tract disease | pathwayBased | 0.205966892490357 | 36 | 376029 | 3978 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | biliary tract disease | targetBased | 0.157007318292117 | 11 | 336894 | 489 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | biliary tract disease | targetBased | 0.21967348408486 | 15 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | biliary tract disease | targetBased | 0.21967348408486 | 15 | 337446 | 1356 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | biliary tract disease | targetBased | 0.167444631881332 | 12 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | biliary tract disease | targetBased | 0.136811743747336 | 8 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | biliary tract disease | pathwayBased | 0.172662097836084 | 8 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | biliary tract disease | pathwayBased | 0.172662097836084 | 8 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | biliary tract disease | targetBased | 0.190422648896797 | 6 | 46715 | 2829 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | esophageal disease | pathwayBased | 0.161486644810321 | 34 | 376029 | 3978 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | biliary tract disease | targetBased | 0.180897290213134 | 43 | 57013 | 201 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | biliary tract disease | targetBased | 0.121412241828512 | 5 | 343468 | 285 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | biliary tract disease | pathwayBased | 0.164530274775241 | 105 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | biliary tract disease | pathwayBased | 0.164530274775241 | 105 | 359244 | 3094 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | biliary tract disease | targetBased | 0.104245002906551 | 14 | 338328 | 2501 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | biliary tract disease | targetBased | 0.20202694721816 | 5 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | biliary tract disease | targetBased | 0.20202694721816 | 5 | 335239 | 695 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | esophageal disease | pathwayBased | 0.102580658913278 | 6 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | esophageal disease | targetBased | 0.150321032541733 | 24 | 336894 | 489 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | esophageal disease | targetBased | 0.116999052702556 | 17 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | esophageal disease | targetBased | 0.116999052702556 | 17 | 290726 | 2649 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | esophageal disease | targetBased | 0.145034469060499 | 4 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | esophageal disease | targetBased | 0.145034469060499 | 4 | 337446 | 1356 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | esophageal disease | targetBased | 0.138614614299397 | 4 | 385746 | 932 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | esophageal disease | targetBased | 0.206900623767966 | 52 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | esophageal disease | targetBased | 0.206900623767966 | 52 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | esophageal disease | targetBased | 0.165730732956527 | 31 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | esophageal disease | targetBased | 0.167853003223802 | 11 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | esophageal disease | pathwayBased | 0.167328604663591 | 17 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | esophageal disease | pathwayBased | 0.167328604663591 | 17 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | esophageal disease | targetBased | 0.139952719795136 | 9 | 46715 | 2829 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | esophageal disease | targetBased | 0.156301703572436 | 50 | 57013 | 201 |
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | esophageal disease | targetBased | 0.177237887811495 | 6 | 564288 | 6030 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | esophageal disease | targetBased | 0.101711451740033 | 38 | 338328 | 2501 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | esophageal disease | targetBased | 0.168042672120834 | 9 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | esophageal disease | targetBased | 0.219137549973724 | 58 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | esophageal disease | pathwayBased | 0.219137549973724 | 58 | 403871 | 1243 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | esophageal disease | targetBased | 0.135230274597126 | 4 | 369953 | 3123 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | esophageal disease | targetBased | 0.134387167760908 | 8 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | esophageal disease | targetBased | 0.216144931591686 | 19 | 344733 | 7043 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | esophageal disease | targetBased | 0.137898774203478 | 35 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | esophageal disease | targetBased | 0.119075651192838 | 8 | 263421 | 615 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | biliary tract disease | targetBased | 0.168611236729175 | 4 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | biliary tract disease | targetBased | 0.181608730458187 | 18 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | biliary tract disease | pathwayBased | 0.181608730458187 | 18 | 403871 | 1243 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | biliary tract disease | targetBased | 0.149830734675932 | 9 | 344733 | 7043 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | biliary tract disease | targetBased | 0.122394134109876 | 69 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | biliary tract disease | targetBased | 0.122394134109876 | 69 | 335180 | 1022 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | biliary tract disease | targetBased | 0.18239531270955 | 27 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | biliary tract disease | targetBased | 0.137721130736681 | 5 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | biliary tract disease | targetBased | 0.148974056748823 | 14 | 207811 | 220 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | RAC1 | biliary tract disease | targetBased | 0.180117774027059 | 5 | 194629 | 219 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | RAC1 | biliary tract disease | targetBased | 0.180117774027059 | 5 | 194628 | 521 |
| E3 Ligase HTS_1536 | MDM2 | esophageal disease | targetBased | 0.166792459560453 | 68 | 207811 | 220 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | esophageal disease | targetBased | 0.136683970652793 | 5 | 47480 | 2010 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | esophageal disease | pathwayBased | 0.286509685530083 | 976 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophageal disease | targetBased | 0.286509685530083 | 976 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophageal disease | targetBased | 0.286509685530083 | 976 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophageal disease | targetBased | 0.286509685530083 | 976 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | esophageal disease | targetBased | 0.286509685530083 | 976 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | esophageal disease | targetBased | 0.14439203425888 | 387 | 356517 | 1139 |
| Inhibitors of CDC25B-CDK2/CyclinA interaction | Inhibitors of CDC25B-CDK2/CyclinA interaction | esophageal disease | targetBased | 0.112949418858417 | 31 | 93798 | 679 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | esophageal disease | targetBased | 0.21142960820566 | 7 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | esophageal disease | targetBased | 0.21142960820566 | 7 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | esophageal disease | targetBased | 0.21142960820566 | 7 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | esophageal disease | targetBased | 0.21142960820566 | 7 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | esophageal disease | targetBased | 0.21142960820566 | 7 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | esophageal disease | targetBased | 0.21142960820566 | 7 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | esophageal disease | targetBased | 0.175103949253225 | 42 | 322361 | 619 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | biliary tract disease | pathwayBased | 0.262109813765427 | 352 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | biliary tract disease | targetBased | 0.262109813765427 | 352 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | biliary tract disease | targetBased | 0.262109813765427 | 352 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | biliary tract disease | targetBased | 0.262109813765427 | 352 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | biliary tract disease | targetBased | 0.262109813765427 | 352 | 124022 | 1156 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | biliary tract disease | targetBased | 0.195725209998113 | 20 | 322361 | 619 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | biliary tract disease | targetBased | 0.122531417380512 | 7 | 325630 | 216 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | biliary tract disease | targetBased | 0.179014143479589 | 4 | 335531 | 328 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | biliary tract disease | targetBased | 0.141106864481345 | 11 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | biliary tract disease | targetBased | 0.141106864481345 | 11 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | biliary tract disease | targetBased | 0.236781323817186 | 19 | 71016 | 346 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | esophageal disease | targetBased | 0.173560529730411 | 4 | 335531 | 328 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | esophageal disease | targetBased | 0.153695536467353 | 15 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | esophageal disease | targetBased | 0.153695536467353 | 15 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | esophageal disease | targetBased | 0.166506375767279 | 6 | 71016 | 346 |
| uHTS for 14-3-3/Bad interaction inhibitors | YWHAZ | esophageal disease | targetBased | 0.102570759914913 | 7 | 217332 | 1549 |
| qHTS for Inhibitors of WRN Helicase | WRN | esophageal disease | targetBased | 0.191754715622543 | 7 | 364011 | 1678 |
| HTS of Smad transcription factor inhibitors | SMAD3 | esophageal disease | targetBased | 0.194916238556379 | 22 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | esophageal disease | targetBased | 0.192213291090885 | 53 | 193542 | 587 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | esophageal disease | pathwayBased | 0.162360550737552 | 170 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | esophageal disease | pathwayBased | 0.162360550737552 | 170 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | esophageal disease | targetBased | 0.191600561848195 | 5 | 339297 | 1446 |
| HTS of Smad transcription factor inhibitors | SMAD3 | biliary tract disease | targetBased | 0.207917987866813 | 8 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | biliary tract disease | targetBased | 0.231001266551921 | 38 | 193542 | 587 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | esophageal disease | targetBased | 0.171768506342496 | 12 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | esophageal disease | targetBased | 0.135165226270177 | 7 | 218654 | 8241 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | biliary tract disease | pathwayBased | 0.204528347323803 | 95 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | biliary tract disease | pathwayBased | 0.204528347323803 | 95 | 194666 | 1722 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | biliary tract disease | targetBased | 0.139045987937789 | 8 | 194628 | 267 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | biliary tract disease | targetBased | 0.179871714145841 | 5 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | biliary tract disease | targetBased | 0.179871714145841 | 5 | 331360 | 2760 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | esophageal disease | targetBased | 0.19148661159607 | 5 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | esophageal disease | targetBased | 0.19148661159607 | 5 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | esophageal disease | targetBased | 0.195319806594737 | 134 | 195821 | 1625 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | esophageal disease | targetBased | 0.187878760757735 | 7 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | esophageal disease | targetBased | 0.142872648633855 | 6 | 331671 | 10022 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | esophageal disease | pathwayBased | 0.198632349484476 | 156 | 43989 | 342 |
| HCS assay for microtubule stabilizers | TUBB | biliary tract disease | targetBased | 0.135387301050889 | 20 | 195821 | 1625 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | biliary tract disease | targetBased | 0.135766365463072 | 5 | 316642 | 617 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | biliary tract disease | targetBased | 0.148693854192137 | 4 | 347933 | 673 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | biliary tract disease | pathwayBased | 0.189230377457323 | 84 | 43989 | 342 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | disease of peritoneum | targetBased | 0.143502261837141 | 4 | 344733 | 7043 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | disease of peritoneum | targetBased | 0.131520183275296 | 12 | 322361 | 619 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | disease of peritoneum | targetBased | 0.239715581954269 | 84 | 71016 | 346 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | disease of peritoneum | targetBased | 0.143162950147737 | 6 | 193542 | 587 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | disorder of appendix | targetBased | 0.168840003758333 | 8 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | disorder of appendix | targetBased | 0.168840003758333 | 8 | 337446 | 1356 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | biliary tract disease | pathwayBased | 0.132135322967985 | 4 | 358556 | 3721 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | biliary tract disease | targetBased | 0.178777478994521 | 4 | 309684 | 1662 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | biliary tract disease | targetBased | 0.174730860546456 | 7 | 47480 | 2010 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | biliary tract disease | targetBased | 0.153287307285792 | 215 | 356517 | 1139 |
| HCS assay for microtubule stabilizers | TUBB | disease of peritoneum | targetBased | 0.193517983302583 | 81 | 195821 | 1625 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | disorder of appendix | pathwayBased | 0.143104560131717 | 12 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of appendix | targetBased | 0.143104560131717 | 12 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of appendix | targetBased | 0.143104560131717 | 12 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of appendix | targetBased | 0.143104560131717 | 12 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | disorder of appendix | targetBased | 0.143104560131717 | 12 | 124022 | 1156 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | disorder of appendix | targetBased | 0.134712522928826 | 5 | 193542 | 587 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | stomach disease | pathwayBased | 0.24963796132147 | 37 | 376029 | 3978 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | stomach disease | targetBased | 0.118265588402072 | 9 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | stomach disease | targetBased | 0.118265588402072 | 9 | 290726 | 2649 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | stomach disease | targetBased | 0.232891928579322 | 19 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | stomach disease | targetBased | 0.232891928579322 | 19 | 337446 | 1356 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | stomach disease | targetBased | 0.169316445076582 | 13 | 217959 | 2390 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | stomach disease | pathwayBased | 0.157745433073832 | 10 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | stomach disease | pathwayBased | 0.157745433073832 | 10 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | stomach disease | targetBased | 0.178472726512756 | 4 | 46715 | 2829 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | stomach disease | targetBased | 0.176628715733264 | 4 | 309684 | 1662 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | stomach disease | targetBased | 0.101027082506027 | 18 | 338328 | 2501 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | stomach disease | targetBased | 0.183415657748161 | 7 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | stomach disease | targetBased | 0.147472481844127 | 14 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | stomach disease | pathwayBased | 0.147472481844127 | 14 | 403871 | 1243 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | stomach disease | targetBased | 0.195970305700043 | 11 | 369953 | 3123 |
| HTS for PAX8 inhibitors using PAX8 luciferase reporter gene assay in RMG-I cells Measured in Cell-Based System Using Plate Reader - 7054-01_Inhibitor_SinglePoint_HTS_Activity | PAX8 | stomach disease | targetBased | 0.100798033713982 | 8 | 353950 | 4145 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | stomach disease | targetBased | 0.129533965903405 | 5 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | stomach disease | targetBased | 0.206663300832383 | 12 | 344733 | 7043 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | stomach disease | targetBased | 0.146910492794997 | 30 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | stomach disease | targetBased | 0.149048734001694 | 16 | 263421 | 615 |
| Inhibitors of CDC25B-CDK2/CyclinA interaction | Inhibitors of CDC25B-CDK2/CyclinA interaction | stomach disease | targetBased | 0.103354433927447 | 10 | 93798 | 679 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | stomach disease | targetBased | 0.222727013425377 | 23 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | stomach disease | targetBased | 0.222727013425377 | 23 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | stomach disease | targetBased | 0.222727013425377 | 23 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | stomach disease | targetBased | 0.222727013425377 | 23 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | stomach disease | targetBased | 0.222727013425377 | 23 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | stomach disease | targetBased | 0.222727013425377 | 23 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | stomach disease | targetBased | 0.270257833247462 | 75 | 322361 | 619 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | stomach disease | targetBased | 0.200094570283736 | 6 | 335531 | 328 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | rectal disease | pathwayBased | 0.101557190330656 | 5 | 376029 | 3978 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | rectal disease | targetBased | 0.180921546531868 | 5 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | rectal disease | targetBased | 0.180921546531868 | 5 | 337446 | 1356 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | rectal disease | targetBased | 0.136875731133718 | 7 | 359207 | 1432 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | rectal disease | targetBased | 0.21158097143505 | 5 | 196177 | 519 |
| E3 Ligase HTS_1536 | MDM2 | rectal disease | targetBased | 0.101856600228584 | 4 | 207811 | 220 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | rectal disease | pathwayBased | 0.229811136836429 | 96 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | rectal disease | targetBased | 0.229811136836429 | 96 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | rectal disease | targetBased | 0.229811136836429 | 96 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | rectal disease | targetBased | 0.229811136836429 | 96 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | rectal disease | targetBased | 0.229811136836429 | 96 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | rectal disease | targetBased | 0.108339187697906 | 10 | 356517 | 1139 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | stomach disease | targetBased | 0.143350120382454 | 4 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | stomach disease | targetBased | 0.143350120382454 | 4 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | stomach disease | targetBased | 0.206902840904954 | 6 | 71016 | 346 |
| qHTS for Inhibitors of WRN Helicase | WRN | stomach disease | targetBased | 0.203454333259954 | 6 | 364011 | 1678 |
| Antagonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | stomach disease | targetBased | 0.107093572753146 | 4 | 329153 | 670 |
| Inverse Agonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | stomach disease | targetBased | 0.107093572753146 | 4 | 329153 | 670 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor | TSHR | stomach disease | targetBased | 0.107093572753146 | 4 | 72026 | 352 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor: Activators of Intracellular cAMP Concentrations in Parental HEK 293 | TSHR | stomach disease | targetBased | 0.107093572753146 | 4 | 72026 | 1794 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | stomach disease | targetBased | 0.242010389540166 | 63 | 193542 | 587 |
| Fluorescence Cell-Free Homogeneous Primary HTS to Identify Inhibitors of Histone Deacetylase 3 | HDAC3 | stomach disease | targetBased | 0.100998221732829 | 8 | 318291 | 483 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | stomach disease | targetBased | 0.186609516030956 | 22 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | stomach disease | targetBased | 0.127030318611918 | 5 | 218654 | 8241 |
| HCS assay for microtubule stabilizers | TUBB | stomach disease | targetBased | 0.209951398343354 | 180 | 195821 | 1625 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | stomach disease | targetBased | 0.164281296415801 | 6 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | stomach disease | targetBased | 0.204655561355192 | 10 | 331671 | 10022 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | rectal disease | targetBased | 0.118916490657654 | 6 | 322361 | 619 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | rectal disease | targetBased | 0.166896834163947 | 9 | 193542 | 587 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | rectal disease | pathwayBased | 0.101935018817883 | 4 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | rectal disease | pathwayBased | 0.101935018817883 | 4 | 194666 | 1722 |
| HCS assay for microtubule stabilizers | TUBB | rectal disease | targetBased | 0.107451196092493 | 4 | 195821 | 1625 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | rectal disease | pathwayBased | 0.127933192574665 | 9 | 43989 | 342 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | anus disease | targetBased | 0.195270739656322 | 4 | 196177 | 519 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | anus disease | pathwayBased | 0.163554881450315 | 21 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | anus disease | targetBased | 0.163554881450315 | 21 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | anus disease | targetBased | 0.163554881450315 | 21 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | anus disease | targetBased | 0.163554881450315 | 21 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | anus disease | targetBased | 0.163554881450315 | 21 | 124022 | 1156 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | anus disease | pathwayBased | 0.102559239991565 | 6 | 43989 | 342 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | malabsorption syndrome | pathwayBased | 0.305064373065845 | 280 | 195980 | 5134 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | malabsorption syndrome | targetBased | 0.305064373065845 | 280 | 195980 | 695 |
| HTS for developing T Cell Immune Modulators | ITGAL | gastrointestinal disease | targetBased | 0.104493469637468 | 56 | 326271 | 221 |
| Fluorescence-based primary biochemical high throughput screening assay to identify inhibitors of Protein Phosphatase 5 (PP5). | PPP5C | gastrointestinal disease | targetBased | 0.152766062605734 | 11 | 314999 | 564 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | gastrointestinal disease | pathwayBased | 0.292809800146558 | 674 | 376029 | 3978 |
| Primary cell-based high-throughput screening assay for identification of compounds that inhibit KCNQ1 potassium channels | KCNQ1 | gastrointestinal disease | targetBased | 0.223133199776835 | 279 | 305610 | 3878 |
| Primary cell-based high-throughput screening assay for identification of compounds that potentiate/activate KCNQ1 potassium channels | KCNQ1 | gastrointestinal disease | targetBased | 0.223133199776835 | 279 | 305610 | 1082 |
| Primary Cell-based High Throughput Screening assay for activators of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | gastrointestinal disease | pathwayBased | 0.141306956955236 | 118 | 64908 | 979 |
| Primary Cell-based High Throughput Screening assay for inhibitors of the Retinoic Acid Receptor-related orphan receptor A (RORA) | RORA | gastrointestinal disease | pathwayBased | 0.141306956955236 | 118 | 64908 | 278 |
| uHTS fluorescence polarization assay for the identification of translation initiation inhibitors (PABP) | PABPC1 | gastrointestinal disease | targetBased | 0.16648648861363 | 52 | 290915 | 2982 |
| uHTS identification of microRNA-mediated mRNA deadenylation inhibitors by fluoresence polarization assay | PABPC1 | gastrointestinal disease | targetBased | 0.16648648861363 | 52 | 359244 | 1307 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | gastrointestinal disease | pathwayBased | 0.166880618779784 | 45 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | gastrointestinal disease | targetBased | 0.204634590094032 | 358 | 336894 | 489 |
| Luminescence-based cell-based primary high throughput screening assay to identify activators of the function of SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 2 (SMARCA2, BRM) | SMARCA2 | gastrointestinal disease | targetBased | 0.150268944160655 | 37 | 368927 | 3838 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | gastrointestinal disease | targetBased | 0.143751901397482 | 185 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | gastrointestinal disease | targetBased | 0.143751901397482 | 185 | 290726 | 2649 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 1 (SRC1; NCOA1) | NCOA1 | gastrointestinal disease | targetBased | 0.119606679969425 | 31 | 359206 | 428 |
| Multiplexed high-throughput screen for small molecule regulators of RGS family protein interactions, specifically RGS4-Galphao. | GNAO1 | gastrointestinal disease | targetBased | 0.108316291732967 | 27 | 218528 | 711 |
| Multiplexed high-throughput screen for small molecule regulators of RGS family protein interactions, specifically RGS7-Galphao. | GNAO1 | gastrointestinal disease | targetBased | 0.108316291732967 | 27 | 218528 | 770 |
| Multiplexed high-throughput screen for small molecule regulators of RGS family protein interactions, specifically RGS8-Galphao. | GNAO1 | gastrointestinal disease | targetBased | 0.108316291732967 | 27 | 218528 | 750 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | gastrointestinal disease | targetBased | 0.167755241729264 | 43 | 385746 | 932 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | gastrointestinal disease | targetBased | 0.162741142338214 | 37 | 194152 | 1342 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | gastrointestinal disease | targetBased | 0.285204436351899 | 532 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | gastrointestinal disease | targetBased | 0.26976163988946 | 302 | 359207 | 1432 |
| uHTS identification of modulators of interaction between CendR and NRP-1 using Fluorescence Polarization assay | NRP1 | gastrointestinal disease | targetBased | 0.101245906208585 | 118 | 363840 | 3086 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | gastrointestinal disease | pathwayBased | 0.253949704640608 | 284 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | gastrointestinal disease | pathwayBased | 0.253949704640608 | 284 | 131324 | 544 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | gastrointestinal disease | targetBased | 0.19450731319173 | 44 | 143816 | 859 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | gastrointestinal disease | targetBased | 0.229814187624333 | 129 | 46715 | 2829 |
| qHTS Assay for Inhibitors of the CtBP/E1A Interaction | RBBP8 | gastrointestinal disease | targetBased | 0.209827344915293 | 12 | 335214 | 1652 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | gastrointestinal disease | targetBased | 0.208282586861204 | 38 | 363803 | 2412 |
| Primary cell-based high throughput screening assay to identify inhibitors of kruppel-like factor 5 (KLF5) | KLF5 | gastrointestinal disease | targetBased | 0.173454869391265 | 119 | 290726 | 671 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | gastrointestinal disease | targetBased | 0.229634748916525 | 672 | 57013 | 201 |
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | gastrointestinal disease | targetBased | 0.206531906123968 | 66 | 564288 | 6030 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | gastrointestinal disease | targetBased | 0.244323770856061 | 55 | 343468 | 285 |
| qHTS of IL-2 Activators | IL2 | gastrointestinal disease | targetBased | 0.13316638131151 | 1520 | 364617 | 238 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | gastrointestinal disease | targetBased | 0.225860725229992 | 42 | 309684 | 1662 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | gastrointestinal disease | targetBased | 0.119412984483291 | 302 | 338328 | 2501 |
| uHTS identification of small molecule antagonists of the CCR6 receptor via a luminescent beta-arrestin assay | CCR6_antagonists | gastrointestinal disease | targetBased | 0.128176815139925 | 15 | 340696 | 1654 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | gastrointestinal disease | targetBased | 0.27152326731022 | 115 | 326888 | 645 |
| Small-molecule inhibitors of ST2 (IL1RL1) | IL1RL1 | gastrointestinal disease | targetBased | 0.139330824393564 | 54 | 75924 | 1804 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | gastrointestinal disease | targetBased | 0.255795785917352 | 248 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | gastrointestinal disease | targetBased | 0.283252811812483 | 688 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | gastrointestinal disease | pathwayBased | 0.283252811812483 | 688 | 403871 | 1243 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | gastrointestinal disease | targetBased | 0.208056487509995 | 100 | 363803 | 458 |
| Fluorescence polarization-based cell-based primary high throughput screening assay to identify activators of insulin-degrading enzyme (IDE) | IDE | gastrointestinal disease | targetBased | 0.121023946732718 | 118 | 335239 | 245 |
| Fluorescence polarization-based cell-based primary high throughput screening assay to identify inhibitors of insulin-degrading enzyme (IDE) | IDE | gastrointestinal disease | targetBased | 0.121023946732718 | 118 | 324747 | 1316 |
| uHTS Fluorescent assay for identification of activators of Apaf-1 | APAF1_activators | gastrointestinal disease | targetBased | 0.10102337635462 | 44 | 331671 | 1041 |
| uHTS Fluorescent assay for identification of inhibitors of Apaf-1 | APAF1_inhibitors | gastrointestinal disease | targetBased | 0.10102337635462 | 44 | 331671 | 2353 |
| uHTS identification of Caspase-8 TRAIL sensitizers in a luminesence assay | TNFRSF10B | gastrointestinal disease | targetBased | 0.114523641022307 | 170 | 363840 | 3764 |
| qHTS for Inhibitors of Cell Surface uPA Generation | PLAU | gastrointestinal disease | targetBased | 0.161241965928027 | 230 | 325247 | 1021 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | gastrointestinal disease | targetBased | 0.234158701179551 | 70 | 369953 | 3123 |
| HTS for PAX8 inhibitors using PAX8 luciferase reporter gene assay in RMG-I cells Measured in Cell-Based System Using Plate Reader - 7054-01_Inhibitor_SinglePoint_HTS_Activity | PAX8 | gastrointestinal disease | targetBased | 0.117603972579397 | 93 | 353950 | 4145 |
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | gastrointestinal disease | targetBased | 0.209134318621081 | 32 | 217959 | 771 |
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | gastrointestinal disease | targetBased | 0.209134318621081 | 32 | 217959 | 569 |
| Primary biochemical fluorescence polarization-based high throughput screening assay to identify inhibitors of protein arginine methyltransferase 1 (PRMT1) | PRMT1 | gastrointestinal disease | targetBased | 0.100484571463705 | 70 | 369953 | 4757 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | gastrointestinal disease | targetBased | 0.215728024431536 | 61 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | gastrointestinal disease | targetBased | 0.270614413856281 | 159 | 344733 | 7043 |
| qHTS Assay for Inhibitors of RGS12 GoLoco Motif Activity (Red Fluorophore) | GNAI1 | gastrointestinal disease | targetBased | 0.10404766432437 | 30 | 229888 | 1007 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of STK33 | STK33 | gastrointestinal disease | targetBased | 0.106509542192313 | 16 | 37341 | 2812 |
| Luminescence Cell-Free Homogenous Primary HTS to Identify Inhibitors of Serine/Threonine Kinase 33 Activity | STK33 | gastrointestinal disease | targetBased | 0.106509542192313 | 16 | 321808 | 235 |
| Factor XIIa 1536 HTS | F12_modulation | gastrointestinal disease | targetBased | 0.144260437454582 | 23 | 217430 | 649 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | gastrointestinal disease | targetBased | 0.139634507373663 | 360 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | gastrointestinal disease | targetBased | 0.139634507373663 | 360 | 335180 | 1022 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | gastrointestinal disease | targetBased | 0.288926026114239 | 411 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | gastrointestinal disease | targetBased | 0.248094120744802 | 452 | 207811 | 220 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | RAC1 | gastrointestinal disease | targetBased | 0.228659996214282 | 41 | 194629 | 219 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | RAC1 | gastrointestinal disease | targetBased | 0.228659996214282 | 41 | 194628 | 521 |
| High throughput screening of activators of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | gastrointestinal disease | targetBased | 0.171704020572574 | 84 | 305610 | 382 |
| High throughput screening of inhibitors of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | gastrointestinal disease | targetBased | 0.171704020572574 | 84 | 305610 | 3253 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | gastrointestinal disease | targetBased | 0.228470060484291 | 38 | 47480 | 2010 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | gastrointestinal disease | targetBased | 0.207686559401033 | 235 | 86095 | 1114 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 2 (SRC2; NCOA2) | NCOA2 | gastrointestinal disease | targetBased | 0.239523363654346 | 23 | 368927 | 620 |
| qHTS Assay for NPC1 Promoter Activators | NPC1 | gastrointestinal disease | pathwayBased | 0.134120682573982 | 53 | 320682 | 7575 |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | gastrointestinal disease | targetBased | 0.157911646074333 | 47 | 108286 | 1415 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | gastrointestinal disease | pathwayBased | 0.157733198515103 | 104 | 7671 | 874 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | gastrointestinal disease | targetBased | 0.157733198515103 | 104 | 304060 | 10600 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | gastrointestinal disease | pathwayBased | 0.157733198515103 | 104 | 305439 | 16717 |
| Inhibitors of CDC25B-CDK2/CyclinA interaction | Inhibitors of CDC25B-CDK2/CyclinA interaction | gastrointestinal disease | targetBased | 0.11895388380973 | 221 | 93798 | 679 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | gastrointestinal disease | targetBased | 0.313769186613035 | 558 | 322361 | 619 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-beta3). | PLCB3 | gastrointestinal disease | targetBased | 0.105947328348397 | 12 | 369953 | 662 |
| Total Fluorescence Counterscreen for Inhibitors of the Interaction of Thyroid Hormone Receptor and Steroid Receptor Coregulator 2 | THRB | gastrointestinal disease | targetBased | 0.148200568497349 | 66 | 276265 | 806 |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | gastrointestinal disease | targetBased | 0.22586173169961 | 18 | 364051 | 9106 |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | gastrointestinal disease | targetBased | 0.22586173169961 | 18 | 407539 | 2380 |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | gastrointestinal disease | targetBased | 0.22586173169961 | 18 | 407539 | 2380 |
| QFRET-based biochemical primary high throughput screening assay to identify exosite inhibitors of ADAM17. | ADAM17_inhibitors | gastrointestinal disease | targetBased | 0.263599855350939 | 432 | 369953 | 3080 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | gastrointestinal disease | targetBased | 0.192004778299403 | 143 | 325630 | 216 |
| uHTS Fluorescent assay for identification of inhibitors of hexokinase domain containing I (HKDC1) | HKDC1 | gastrointestinal disease | targetBased | 0.163958011078626 | 37 | 340696 | 540 |
| uHTS of small molecular inhibitors for p47phox, a regulatory protein of NADPH oxidases (Noxs) | p47phoxInhibitors | gastrointestinal disease | targetBased | 0.117476139296129 | 73 | 217454 | 1142 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | gastrointestinal disease | targetBased | 0.221403550207962 | 134 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | gastrointestinal disease | targetBased | 0.221403550207962 | 134 | 218234 | 1620 |
| qHTS for PTHR1 Agonists: Primary Screen | PTH1R | gastrointestinal disease | targetBased | 0.117243469330359 | 17 | 405685 | 308 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | gastrointestinal disease | targetBased | 0.287120827849473 | 163 | 71016 | 346 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | gastrointestinal disease | targetBased | 0.162149690155714 | 71 | 148480 | 6428 |
| MLPCN ERAP1 Measured in Biochemical System Using Plate Reader - 7016-01_Inhibitor_SinglePoint_HTS_Activity | ERAP1_inhibitors | gastrointestinal disease | targetBased | 0.168938956280752 | 33 | 335777 | 499 |
| Fluorescence-based primary biochemical high throughput screening assay to identify inhibitors of human diacylglycerol lipase, beta (DAGLB) | DAGLB_inhibitors | gastrointestinal disease | targetBased | 0.137754738715669 | 5 | 343468 | 202 |
| uHTS for 14-3-3/Bad interaction inhibitors | YWHAZ | gastrointestinal disease | targetBased | 0.118646632056597 | 71 | 217332 | 1549 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of HIV-1 LEDGF/p75 DNA Integration | PSIP1 | gastrointestinal disease | targetBased | 0.217645116160563 | 51 | 369953 | 2353 |
| qHTS for Inhibitors of WRN Helicase | WRN | gastrointestinal disease | targetBased | 0.284615917758805 | 58 | 364011 | 1678 |
| Antagonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | gastrointestinal disease | targetBased | 0.185913171224615 | 44 | 329153 | 670 |
| Inverse Agonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | gastrointestinal disease | targetBased | 0.185913171224615 | 44 | 329153 | 670 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor | TSHR | gastrointestinal disease | targetBased | 0.185913171224615 | 44 | 72026 | 352 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor: Activators of Intracellular cAMP Concentrations in Parental HEK 293 | TSHR | gastrointestinal disease | targetBased | 0.185913171224615 | 44 | 72026 | 1794 |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | gastrointestinal disease | targetBased | 0.211336087890814 | 15 | 324747 | 813 |
| Luminescence-based cell-based primary high throughput screening assay to identify biased ligands of the melanocortin 4 receptor (MC4R): agonists of MC4R | MC4R | gastrointestinal disease | targetBased | 0.219902537409309 | 261 | 356160 | 3470 |
| TRFRET-based cell-based primary high throughput screening assay to identify biased ligands of the melanocortin 4 receptor (MC4R): antagonists of MC4R | MC4R | gastrointestinal disease | targetBased | 0.219902537409309 | 261 | 356160 | 1703 |
| HTS of Smad transcription factor inhibitors | SMAD3 | gastrointestinal disease | targetBased | 0.2981091823051 | 433 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | gastrointestinal disease | targetBased | 0.31768133228557 | 1458 | 193542 | 587 |
| Fluorescence polarization-based primary biochemical high throughput screening assay to identify inhibitors of human platelet-activating factor acetylhydrolase 1b, catalytic subunit 2 (PAFAH1B2) | PAFAH1B2 | gastrointestinal disease | targetBased | 0.121417616781695 | 16 | 335239 | 4158 |
| qHTS Assay for the Inhibitors of Human Flap endonuclease 1 (FEN1). | FEN1_inhibitors | gastrointestinal disease | targetBased | 0.120864842358742 | 58 | 386270 | 1331 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | gastrointestinal disease | targetBased | 0.190921641975683 | 14 | 305610 | 3794 |
| OTUD3 deubiquitinase inhibition: Primary qHTS | OTUD3 | gastrointestinal disease | targetBased | 0.136463448283477 | 33 | 47480 | 334 |
| HTS for 14-3-3 protein interaction modulators | YWHAG | gastrointestinal disease | targetBased | 0.104766970349099 | 13 | 157962 | 312 |
| qHTS Assay for Activators of Human alpha-Glucosidase as a Potential Chaperone Treatment of Pompe Disease | GAA | gastrointestinal disease | targetBased | 0.21462068957783 | 57 | 199169 | 715 |
| qHTS Assay for Inhibitors and Activators of Human alpha-Glucosidase Cleavage of Glycogen | GAA_inhibitors | gastrointestinal disease | targetBased | 0.21462068957783 | 57 | 302297 | 1165 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK3 | KCNK3 | gastrointestinal disease | targetBased | 0.184873422701528 | 11 | 339674 | 2841 |
| Fluorescence Cell-Free Homogeneous Primary HTS to Identify Inhibitors of Histone Deacetylase 3 | HDAC3 | gastrointestinal disease | targetBased | 0.10751628289169 | 182 | 318291 | 483 |
| Inhibition of the MLL-AF4-AF9 Interaction in Pediatric Leukemia Measured in Biochemical System Using Plate Reader - 2160-01_Inhibitor_SinglePoint_HTS_Activity | MLLT3 | gastrointestinal disease | targetBased | 0.130180356805543 | 20 | 344459 | 1627 |
| SMM ID4 Measured in Biochemical System Using Small Molecule MicroArray - 2128-01_Other_SinglePoint_HTS_Activity | ID4 | gastrointestinal disease | targetBased | 0.16367620589902 | 19 | 31324 | 362 |
| qHTS for Small Molecule Agonists and Allosteric Enhancers of Human TRH Receptor: Primary Screen for Agonists. | TRHR | gastrointestinal disease | targetBased | 0.144569987528856 | 4 | 361330 | 651 |
| qHTS for Small Molecule Agonists and Allosteric Enhancers of Human TRH Receptor: Primary Screen for Enhancers | TRHR | gastrointestinal disease | targetBased | 0.144569987528856 | 4 | 361330 | 2424 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | gastrointestinal disease | targetBased | 0.265204098812013 | 327 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | gastrointestinal disease | targetBased | 0.172930043009415 | 378 | 218654 | 8241 |
| Absorbance-based biochemical primary high throughput screening assay to identify activators of Methionine sulfoxide reductase A (MsrA) | MSRA | gastrointestinal disease | targetBased | 0.165631221932114 | 8 | 362026 | 1074 |
| Absorbance-based biochemical primary high throughput screening assay to identify inhibitors of Methionine sulfoxide reductase A (MsrA) | MSRA | gastrointestinal disease | targetBased | 0.165631221932114 | 8 | 362577 | 2709 |
| Glucocerebrosidase | GBA1 | gastrointestinal disease | targetBased | 0.106079421835122 | 74 | 48118 | 549 |
| uHTS identification of UBC13 Polyubiquitin Inhibitors via a TR-FRET Assay | UBE2N | gastrointestinal disease | targetBased | 0.10313700521933 | 13 | 330393 | 1538 |
| Assay for HTS of Gi/Go-linked GPCRs using mGluR8: Primary Screening | GRM8 | gastrointestinal disease | targetBased | 0.151908351849933 | 7 | 105151 | 2166 |
| Thrombin 1536 HTS | F2_modulation | gastrointestinal disease | targetBased | 0.186037512198385 | 694 | 217233 | 557 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | gastrointestinal disease | targetBased | 0.260881738372858 | 91 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | gastrointestinal disease | targetBased | 0.260881738372858 | 91 | 331360 | 2760 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | gastrointestinal disease | targetBased | 0.259300711784485 | 44 | 347933 | 673 |
| Alphascreen assay for small molecules abrogating mHTT-CaM Interaction | HTT | gastrointestinal disease | targetBased | 0.111384780455362 | 18 | 189882 | 6790 |
| Primary qHTS for Identification of Small Molecule Inhibitors of Huntingtin Promoter Activity | HTT | gastrointestinal disease | targetBased | 0.111384780455362 | 18 | 48068 | 449 |
| qHTS Multiplex Assay to Identify Dual Action Probes in a Cell Model of Huntington: Aggregate Formation (GFP) | HTT | gastrointestinal disease | targetBased | 0.111384780455362 | 18 | 220571 | 2380 |
| qHTS Multiplex Assay to Identify Dual Action Probes in a Cell Model of Huntington: Cytoprotection (ATP) | HTT | gastrointestinal disease | targetBased | 0.111384780455362 | 18 | 223611 | 305 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | gastrointestinal disease | targetBased | 0.25817151429536 | 56 | 331671 | 10022 |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | gastrointestinal disease | targetBased | 0.213648626685929 | 11 | 339772 | 8480 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | gastrointestinal disease | pathwayBased | 0.27437888372023 | 2809 | 43989 | 342 |
| Primary qHTS for Inhibitors of ATXN expression | ATXN2_repressors | gastrointestinal disease | targetBased | 0.135047408448522 | 55 | 358434 | 2554 |
| Primary HTS Assay for S1P3 Antagonists | S1PR3 | gastrointestinal disease | targetBased | 0.154644535758242 | 19 | 169141 | 462 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | gastrointestinal disease | targetBased | 0.227657848733391 | 528 | 343468 | 734 |
| Primary screen for compounds that activate Insulin promoter activity in TRM-6 cells | INS | gastrointestinal disease | targetBased | 0.320960159387738 | 2595 | 128695 | 1039 |
| Primary screen for compounds that inhibit Insulin promoter activity in TRM-6 cells | INS | gastrointestinal disease | targetBased | 0.320960159387738 | 2595 | 127961 | 1153 |
| Primary Cell-Based High-Throughput Screening to Identify Agonists of the Sphingosine 1-phosphate receptor 2 (S1P2) | S1PR2 | gastrointestinal disease | targetBased | 0.160793537164115 | 78 | 96879 | 447 |
| Primary Cell-Based High-Throughput Screening to Identify Antagonists of the Sphingosine 1-phosphate receptor 2 (S1P2) | S1PR2 | gastrointestinal disease | targetBased | 0.160793537164115 | 78 | 96879 | 207 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | hepatobiliary disease | pathwayBased | 0.233299171288249 | 100 | 376029 | 3978 |
| uHTS fluorescence polarization assay for the identification of translation initiation inhibitors (PABP) | PABPC1 | hepatobiliary disease | targetBased | 0.163197725468031 | 20 | 290915 | 2982 |
| uHTS identification of microRNA-mediated mRNA deadenylation inhibitors by fluoresence polarization assay | PABPC1 | hepatobiliary disease | targetBased | 0.163197725468031 | 20 | 359244 | 1307 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | hepatobiliary disease | pathwayBased | 0.154245443760798 | 17 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | hepatobiliary disease | targetBased | 0.192831865392446 | 168 | 336894 | 489 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | hepatobiliary disease | targetBased | 0.111967832806084 | 69 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | hepatobiliary disease | targetBased | 0.111967832806084 | 69 | 290726 | 2649 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | hepatobiliary disease | targetBased | 0.153521442408727 | 11 | 290355 | 265 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 1 (SRC1; NCOA1) | NCOA1 | hepatobiliary disease | targetBased | 0.110597314863047 | 10 | 359206 | 428 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | hepatobiliary disease | targetBased | 0.155249960083543 | 21 | 385746 | 932 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | hepatobiliary disease | targetBased | 0.105079423077969 | 14 | 194152 | 1342 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | hepatobiliary disease | targetBased | 0.243335422522965 | 352 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | hepatobiliary disease | targetBased | 0.243335422522965 | 352 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | hepatobiliary disease | targetBased | 0.203893104985258 | 193 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | hepatobiliary disease | targetBased | 0.186799141278052 | 55 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | hepatobiliary disease | pathwayBased | 0.211260909234671 | 110 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | hepatobiliary disease | pathwayBased | 0.211260909234671 | 110 | 131324 | 544 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | hepatobiliary disease | targetBased | 0.128684420801562 | 16 | 143816 | 859 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | hepatobiliary disease | targetBased | 0.207500951071491 | 51 | 46715 | 2829 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | hepatobiliary disease | targetBased | 0.163530826036249 | 14 | 363803 | 2412 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | hepatobiliary disease | targetBased | 0.195647925547466 | 371 | 57013 | 201 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | hepatobiliary disease | targetBased | 0.15033107261082 | 11 | 343468 | 285 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | hepatobiliary disease | pathwayBased | 0.173550674308779 | 1849 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | hepatobiliary disease | pathwayBased | 0.173550674308779 | 1849 | 359244 | 3094 |
| qHTS of IL-2 Activators | IL2 | hepatobiliary disease | targetBased | 0.117045934599004 | 444 | 364617 | 238 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | hepatobiliary disease | targetBased | 0.208124287636066 | 12 | 309684 | 1662 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | hepatobiliary disease | targetBased | 0.113792748592726 | 97 | 338328 | 2501 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | hepatobiliary disease | targetBased | 0.228746590466616 | 163 | 394050 | 3624 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | hepatobiliary disease | targetBased | 0.101140056534914 | 1542 | 67275 | 2509 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | hepatobiliary disease | targetBased | 0.215871940867625 | 24 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | hepatobiliary disease | targetBased | 0.215871940867625 | 24 | 335239 | 695 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | hepatobiliary disease | targetBased | 0.204483646617692 | 401 | 405130 | 6428 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | hepatobiliary disease | targetBased | 0.204483646617692 | 401 | 373462 | 23 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | hepatobiliary disease | targetBased | 0.204483646617692 | 401 | 405130 | 6428 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | hepatobiliary disease | targetBased | 0.208029096495953 | 129 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | hepatobiliary disease | targetBased | 0.266173945715519 | 278 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | hepatobiliary disease | pathwayBased | 0.266173945715519 | 278 | 403871 | 1243 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | hepatobiliary disease | targetBased | 0.122376234550443 | 27 | 363803 | 458 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | hepatobiliary disease | targetBased | 0.160353525938876 | 21 | 369953 | 3123 |
| HTS for PAX8 inhibitors using PAX8 luciferase reporter gene assay in RMG-I cells Measured in Cell-Based System Using Plate Reader - 7054-01_Inhibitor_SinglePoint_HTS_Activity | PAX8 | hepatobiliary disease | targetBased | 0.108541418108503 | 18 | 353950 | 4145 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | hepatobiliary disease | targetBased | 0.190711597413071 | 33 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | hepatobiliary disease | targetBased | 0.212328713611818 | 58 | 344733 | 7043 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | hepatobiliary disease | targetBased | 0.122786576361256 | 85 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | hepatobiliary disease | targetBased | 0.122786576361256 | 85 | 335180 | 1022 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | hepatobiliary disease | targetBased | 0.167329973608451 | 21 | 263421 | 615 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | RAC1 | hepatobiliary disease | targetBased | 0.207821447045824 | 16 | 194629 | 219 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | RAC1 | hepatobiliary disease | targetBased | 0.207821447045824 | 16 | 194628 | 521 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | hepatobiliary disease | targetBased | 0.213297210219203 | 15 | 47480 | 2010 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | hepatobiliary disease | targetBased | 0.191533238653504 | 47 | 86095 | 1114 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 2 (SRC2; NCOA2) | NCOA2 | hepatobiliary disease | targetBased | 0.205775427659954 | 8 | 368927 | 620 |
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | hepatobiliary disease | targetBased | 0.135711779783981 | 6 | 108286 | 1415 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | hepatobiliary disease | targetBased | 0.140703132158959 | 11 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | hepatobiliary disease | targetBased | 0.24126381887999 | 100 | 322361 | 619 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | hepatobiliary disease | targetBased | 0.130003212633344 | 61 | 325630 | 216 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | hepatobiliary disease | targetBased | 0.243264311857587 | 9 | 335531 | 328 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of HIV-1 LEDGF/p75 DNA Integration | PSIP1 | hepatobiliary disease | targetBased | 0.18805189787513 | 6 | 369953 | 2353 |
| qHTS for Inhibitors of WRN Helicase | WRN | hepatobiliary disease | targetBased | 0.194949916299053 | 6 | 364011 | 1678 |
| Antagonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | hepatobiliary disease | targetBased | 0.104539783439416 | 13 | 329153 | 670 |
| Inverse Agonists of the Thyroid Stimulating Hormone Receptor: HTS campaign | TSHR | hepatobiliary disease | targetBased | 0.104539783439416 | 13 | 329153 | 670 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor | TSHR | hepatobiliary disease | targetBased | 0.104539783439416 | 13 | 72026 | 352 |
| qHTS Assay for Agonists of the Thyroid Stimulating Hormone Receptor: Activators of Intracellular cAMP Concentrations in Parental HEK 293 | TSHR | hepatobiliary disease | targetBased | 0.104539783439416 | 13 | 72026 | 1794 |
| HTS of Smad transcription factor inhibitors | SMAD3 | hepatobiliary disease | targetBased | 0.232536282971102 | 173 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | hepatobiliary disease | targetBased | 0.304072292572626 | 779 | 193542 | 587 |
| qHTS Assay for the Inhibitors of Human Flap endonuclease 1 (FEN1). | FEN1_inhibitors | hepatobiliary disease | targetBased | 0.109055770299879 | 28 | 386270 | 1331 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | hepatobiliary disease | pathwayBased | 0.230074143610555 | 1252 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | hepatobiliary disease | pathwayBased | 0.230074143610555 | 1252 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | hepatobiliary disease | targetBased | 0.218722620378742 | 30 | 339297 | 1446 |
| Primary cell-based screen for identification of compounds that inhibit the two-pore domain potassium channel KCNK9 | KCNK9_blockers | hepatobiliary disease | targetBased | 0.173225499220873 | 4 | 305610 | 3794 |
| Inhibition of the MLL-AF4-AF9 Interaction in Pediatric Leukemia Measured in Biochemical System Using Plate Reader - 2160-01_Inhibitor_SinglePoint_HTS_Activity | MLLT3 | hepatobiliary disease | targetBased | 0.101919235435679 | 6 | 344459 | 1627 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | hepatobiliary disease | targetBased | 0.190911839025298 | 148 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | hepatobiliary disease | targetBased | 0.151844513368892 | 159 | 218654 | 8241 |
| Glucocerebrosidase | GBA1 | hepatobiliary disease | targetBased | 0.104183798486477 | 41 | 48118 | 549 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | hepatobiliary disease | targetBased | 0.21282684832897 | 25 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | hepatobiliary disease | targetBased | 0.21282684832897 | 25 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | hepatobiliary disease | targetBased | 0.201138703475521 | 35 | 195821 | 1625 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | hepatobiliary disease | targetBased | 0.206853606983743 | 33 | 316642 | 617 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | hepatobiliary disease | targetBased | 0.195082888658765 | 8 | 347933 | 673 |
| uHTS identification of small molecule modulators of NR3A | GRIN3A | hepatobiliary disease | targetBased | 0.196225566665494 | 6 | 339772 | 8480 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | hepatobiliary disease | pathwayBased | 0.219338540178192 | 1351 | 43989 | 342 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | hepatobiliary disease | targetBased | 0.216728630177879 | 233 | 343468 | 734 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of the interaction of nucleotide-binding oligomerization domain containing 2 (NOD2) and the receptor-interacting serine-threonine kinase 2 (RIPK2) | RIPK2 | gastrointestinal disease | targetBased | 0.156703055623001 | 60 | 363803 | 1383 |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | gastrointestinal disease | targetBased | 0.231220365627381 | 140 | 55710 | 315 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | hepatobiliary disease | targetBased | 0.252559439624571 | 49 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | hepatobiliary disease | targetBased | 0.252559439624571 | 49 | 337446 | 1356 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | hepatobiliary disease | targetBased | 0.182662456937424 | 2775 | 356517 | 1139 |
| Total Fluorescence Counterscreen for Inhibitors of the Interaction of Thyroid Hormone Receptor and Steroid Receptor Coregulator 2 | THRB | hepatobiliary disease | targetBased | 0.137568768069957 | 49 | 276265 | 806 |
| uHTS Fluorescent assay for identification of inhibitors of hexokinase domain containing I (HKDC1) | HKDC1 | hepatobiliary disease | targetBased | 0.160741937145607 | 18 | 340696 | 540 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | hepatobiliary disease | targetBased | 0.198801193486026 | 56 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | hepatobiliary disease | targetBased | 0.198801193486026 | 56 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | hepatobiliary disease | targetBased | 0.249782482673105 | 31 | 71016 | 346 |
| Absorbance-based primary biochemical high throughput screening assay to identify activators of procaspase-3 | procaspase3Activators | hepatobiliary disease | targetBased | 0.105087811810551 | 617 | 326024 | 350 |
| MLPCN ERAP1 Measured in Biochemical System Using Plate Reader - 7016-01_Inhibitor_SinglePoint_HTS_Activity | ERAP1_inhibitors | hepatobiliary disease | targetBased | 0.11912117153766 | 7 | 335777 | 499 |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | hepatobiliary disease | targetBased | 0.207881041819684 | 11 | 324747 | 813 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | hepatobiliary disease | targetBased | 0.205839360705494 | 18 | 331671 | 10022 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | mouth disease | pathwayBased | 0.152611065952 | 16 | 376029 | 3978 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | mouth disease | pathwayBased | 0.122478854644805 | 6 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | mouth disease | targetBased | 0.140515721820692 | 16 | 336894 | 489 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | mouth disease | targetBased | 0.141860656424844 | 7 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | mouth disease | targetBased | 0.141860656424844 | 7 | 337446 | 1356 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | mouth disease | targetBased | 0.137043999214659 | 32 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | mouth disease | targetBased | 0.155635908071394 | 6 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | mouth disease | pathwayBased | 0.135934069051813 | 13 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | mouth disease | pathwayBased | 0.135934069051813 | 13 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | mouth disease | targetBased | 0.141229320869979 | 8 | 46715 | 2829 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | mouth disease | targetBased | 0.188762530959518 | 58 | 57013 | 201 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | mouth disease | targetBased | 0.222452538352194 | 17 | 343468 | 285 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | mouth disease | targetBased | 0.195791921694685 | 8 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | mouth disease | targetBased | 0.195791921694685 | 8 | 335239 | 695 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | mouth disease | targetBased | 0.130503465707649 | 8 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | mouth disease | targetBased | 0.1809801953265 | 54 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | mouth disease | pathwayBased | 0.1809801953265 | 54 | 403871 | 1243 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | mouth disease | targetBased | 0.129719180225366 | 6 | 369953 | 3123 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | mouth disease | targetBased | 0.119348171793671 | 4 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | mouth disease | targetBased | 0.156905161179501 | 6 | 344733 | 7043 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | mouth disease | targetBased | 0.114749040297401 | 39 | 196177 | 519 |
| E3 Ligase HTS_1536 | MDM2 | mouth disease | targetBased | 0.128922285654411 | 49 | 207811 | 220 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | mouth disease | targetBased | 0.158455586552102 | 23 | 322361 | 619 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | mouth disease | targetBased | 0.159660561892357 | 11 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | mouth disease | targetBased | 0.159660561892357 | 11 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | mouth disease | targetBased | 0.141921681169004 | 4 | 71016 | 346 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | mouth disease | targetBased | 0.202945655379324 | 52 | 193542 | 587 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | mouth disease | pathwayBased | 0.148394046074413 | 158 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | mouth disease | pathwayBased | 0.148394046074413 | 158 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | mouth disease | targetBased | 0.210608525065713 | 13 | 339297 | 1446 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | mouth disease | targetBased | 0.243574122274198 | 53 | 194628 | 267 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | mouth disease | targetBased | 0.140370872375495 | 7 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | mouth disease | targetBased | 0.140370872375495 | 7 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | mouth disease | targetBased | 0.142666772845746 | 24 | 195821 | 1625 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | mouth disease | targetBased | 0.17910282870345 | 38 | 316642 | 617 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | mouth disease | pathwayBased | 0.157141739120913 | 175 | 43989 | 342 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | mouth disease | targetBased | 0.189544391571219 | 44 | 343468 | 734 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | mouth disease | targetBased | 0.134566651280402 | 10 | 309684 | 1662 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | gastroenteritis | targetBased | 0.154633806653938 | 7 | 194152 | 1342 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | gastroenteritis | targetBased | 0.218506072992008 | 26 | 326888 | 645 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | gastroenteritis | targetBased | 0.173203417600584 | 9 | 363803 | 458 |
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | gastroenteritis | targetBased | 0.196694290124942 | 12 | 217959 | 771 |
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | gastroenteritis | targetBased | 0.196694290124942 | 12 | 217959 | 569 |
| HTS of Smad transcription factor inhibitors | SMAD3 | gastroenteritis | targetBased | 0.16044344143482 | 28 | 88033 | 251 |
| OTUD3 deubiquitinase inhibition: Primary qHTS | OTUD3 | gastroenteritis | targetBased | 0.114483639503231 | 14 | 47480 | 334 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | gastroenteritis | targetBased | 0.110253624464568 | 80 | 394050 | 3624 |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | gastroenteritis | targetBased | 0.214171489449838 | 52 | 55710 | 315 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | Abnormal abdomen morphology | targetBased | 0.225725453668566 | 99 | 217959 | 2390 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Abnormal intestine morphology | pathwayBased | 0.121772986122181 | 4 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal intestine morphology | targetBased | 0.121772986122181 | 4 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal intestine morphology | targetBased | 0.121772986122181 | 4 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal intestine morphology | targetBased | 0.121772986122181 | 4 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal intestine morphology | targetBased | 0.121772986122181 | 4 | 124022 | 1156 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Abnormal gastrointestinal tract morphology | pathwayBased | 0.123067376409661 | 13 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal gastrointestinal tract morphology | targetBased | 0.123067376409661 | 13 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal gastrointestinal tract morphology | targetBased | 0.123067376409661 | 13 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal gastrointestinal tract morphology | targetBased | 0.123067376409661 | 13 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormal gastrointestinal tract morphology | targetBased | 0.123067376409661 | 13 | 124022 | 1156 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | Abnormal gastrointestinal tract morphology | targetBased | 0.207827625009424 | 5 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | Abnormal gastrointestinal tract morphology | targetBased | 0.207827625009424 | 5 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | Abnormal gastrointestinal tract morphology | targetBased | 0.207827625009424 | 5 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | Abnormal gastrointestinal tract morphology | targetBased | 0.207827625009424 | 5 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | Abnormal gastrointestinal tract morphology | targetBased | 0.207827625009424 | 5 | 359207 | 4555 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Functional abnormality of the gastrointestinal tract | targetBased | 0.140147038104654 | 13 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Functional abnormality of the gastrointestinal tract | targetBased | 0.140147038104654 | 13 | 296501 | 2737 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Functional abnormality of the gastrointestinal tract | targetBased | 0.204721142769349 | 5 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Functional abnormality of the gastrointestinal tract | targetBased | 0.204721142769349 | 5 | 335239 | 695 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Functional abnormality of the gastrointestinal tract | targetBased | 0.17251169168788 | 5 | 196177 | 519 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Abnormality of the gastrointestinal tract | targetBased | 0.150809719053129 | 18 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Abnormality of the gastrointestinal tract | targetBased | 0.150809719053129 | 18 | 296501 | 2737 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Abnormality of the gastrointestinal tract | pathwayBased | 0.12524806865127 | 20 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the gastrointestinal tract | targetBased | 0.12524806865127 | 20 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the gastrointestinal tract | targetBased | 0.12524806865127 | 20 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the gastrointestinal tract | targetBased | 0.12524806865127 | 20 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the gastrointestinal tract | targetBased | 0.12524806865127 | 20 | 124022 | 1156 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | Abnormality of the gastrointestinal tract | targetBased | 0.20917999349553 | 5 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | Abnormality of the gastrointestinal tract | targetBased | 0.20917999349553 | 5 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | Abnormality of the gastrointestinal tract | targetBased | 0.20917999349553 | 5 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | Abnormality of the gastrointestinal tract | targetBased | 0.20917999349553 | 5 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | Abnormality of the gastrointestinal tract | targetBased | 0.20917999349553 | 5 | 359207 | 4555 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | Abnormality of the digestive system | targetBased | 0.256738770550522 | 72 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | Abnormality of the digestive system | targetBased | 0.256738770550522 | 72 | 296501 | 2737 |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | Abnormality of the digestive system | targetBased | 0.206711417313871 | 13 | 290355 | 265 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | Abnormality of the digestive system | targetBased | 0.22706601916472 | 131 | 217959 | 2390 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | Abnormality of the digestive system | targetBased | 0.222081786350855 | 46 | 363803 | 2412 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Abnormality of the digestive system | targetBased | 0.222689384826116 | 127 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Abnormality of the digestive system | targetBased | 0.222689384826116 | 127 | 335239 | 695 |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | Abnormality of the digestive system | targetBased | 0.14276286347638 | 129 | 405130 | 6428 |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | Abnormality of the digestive system | targetBased | 0.14276286347638 | 129 | 373462 | 23 |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | Abnormality of the digestive system | targetBased | 0.14276286347638 | 129 | 405130 | 6428 |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | Abnormality of the digestive system | targetBased | 0.203843720207239 | 11 | 306502 | 3633 |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | Abnormality of the digestive system | targetBased | 0.203843720207239 | 11 | 335180 | 1022 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | Abnormality of the digestive system | targetBased | 0.194395799123269 | 302 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | Abnormality of the digestive system | targetBased | 0.126088196007265 | 9 | 263421 | 615 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | Abnormality of the digestive system | pathwayBased | 0.12682763748055 | 137 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the digestive system | targetBased | 0.12682763748055 | 137 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the digestive system | targetBased | 0.12682763748055 | 137 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the digestive system | targetBased | 0.12682763748055 | 137 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | Abnormality of the digestive system | targetBased | 0.12682763748055 | 137 | 124022 | 1156 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | Abnormality of the digestive system | targetBased | 0.220531063776147 | 54 | 336308 | 6862 |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | Abnormality of the digestive system | targetBased | 0.215321018787221 | 24 | 364051 | 9106 |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | Abnormality of the digestive system | targetBased | 0.215321018787221 | 24 | 407539 | 2380 |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | Abnormality of the digestive system | targetBased | 0.215321018787221 | 24 | 407539 | 2380 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | Abnormality of the digestive system | targetBased | 0.130492759443414 | 14 | 325630 | 216 |
| Counterscreen for agonists of OPRM1-OPRD1 heterodimerization: luminescence-based cell-based full-deck high throughput screening assay to identify agonists of 5-hydroxytryptamine (serotonin) 5A receptor (HTR5A) | HTR5A | Abnormality of the digestive system | targetBased | 0.188838073316936 | 19 | 335239 | 2035 |
| Counterscreen for inverse agonists of OPRM1-OPRD1 heterodimerization: luminescence-based cell-based full-deck high throughput screening assay to identify inverse agonists of 5-hydroxytryptamine (serotonin) 5A receptor (HTR5A) | HTR5A | Abnormality of the digestive system | targetBased | 0.188838073316936 | 19 | 335239 | 279 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | Abnormality of the digestive system | targetBased | 0.125466136055478 | 6 | 335531 | 328 |
| Dyrk1 A HTS Measured in Biochemical System Using Plate Reader - 2124-01_Inhibitor_SinglePoint_HTS_Activity | DYRK1A_inhibitors | Abnormality of the digestive system | targetBased | 0.185079044650762 | 4 | 310014 | 1321 |
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | Abnormality of the digestive system | targetBased | 0.201420259688976 | 4 | 324747 | 813 |
| Primary Cell Based High Throughput Screening Assay for Agonists of the 5-Hydroxytryptamine Receptor Subtype 1E (5HT1E) | HTR1E | Abnormality of the digestive system | targetBased | 0.18739589566178 | 4 | 64908 | 446 |
| Primary Cell Based High Throughput Screening Assay for Antagonists of the 5-Hydroxytryptamine Receptor Subtype 1E (5HT1E) | HTR1E | Abnormality of the digestive system | targetBased | 0.18739589566178 | 4 | 64908 | 641 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | Abnormality of the digestive system | targetBased | 0.17687633711971 | 8 | 339297 | 1446 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | Abnormality of the digestive system | targetBased | 0.201490789047156 | 16 | 64908 | 366 |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | Abnormality of the digestive system | targetBased | 0.201490789047156 | 16 | 61606 | 416 |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | Abnormality of the digestive system | targetBased | 0.210513177077567 | 27 | 316642 | 617 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Abnormality of the gastrointestinal tract | targetBased | 0.204721142769349 | 5 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | Abnormality of the gastrointestinal tract | targetBased | 0.204721142769349 | 5 | 335239 | 695 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | Abnormality of the digestive system | pathwayBased | 0.170143595846741 | 45 | 195980 | 5134 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | Abnormality of the digestive system | targetBased | 0.170143595846741 | 45 | 195980 | 695 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | Abnormality of the digestive system | targetBased | 0.216848951258122 | 14 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | Abnormality of the digestive system | targetBased | 0.216848951258122 | 14 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | Abnormality of the digestive system | targetBased | 0.216848951258122 | 14 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | Abnormality of the digestive system | targetBased | 0.216848951258122 | 14 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | Abnormality of the digestive system | targetBased | 0.216848951258122 | 14 | 359207 | 4555 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | cecal disorder | targetBased | 0.19332836794129 | 10 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | cecal disorder | targetBased | 0.19332836794129 | 10 | 337446 | 1356 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | cecal disorder | targetBased | 0.127938133524008 | 5 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | cecal disorder | targetBased | 0.127938133524008 | 5 | 86095 | 1151 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | cecal disorder | pathwayBased | 0.187425053136919 | 16 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | cecal disorder | targetBased | 0.187425053136919 | 16 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | cecal disorder | targetBased | 0.187425053136919 | 16 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | cecal disorder | targetBased | 0.187425053136919 | 16 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | cecal disorder | targetBased | 0.187425053136919 | 16 | 124022 | 1156 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | cecal disorder | targetBased | 0.145496487674544 | 7 | 193542 | 587 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | functional gastric disease | targetBased | 0.212795330133383 | 18 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | functional gastric disease | targetBased | 0.212795330133383 | 18 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | functional gastric disease | targetBased | 0.212795330133383 | 18 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | functional gastric disease | targetBased | 0.212795330133383 | 18 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | functional gastric disease | targetBased | 0.212795330133383 | 18 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | functional gastric disease | targetBased | 0.212795330133383 | 18 | 336308 | 6862 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | duodenal disorder | targetBased | 0.118625969456299 | 4 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | duodenal disorder | targetBased | 0.118625969456299 | 4 | 86095 | 1151 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | duodenal disorder | pathwayBased | 0.128317533150862 | 22 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | duodenal disorder | targetBased | 0.128317533150862 | 22 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | duodenal disorder | targetBased | 0.128317533150862 | 22 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | duodenal disorder | targetBased | 0.128317533150862 | 22 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | duodenal disorder | targetBased | 0.128317533150862 | 22 | 124022 | 1156 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | duodenal disorder | targetBased | 0.12808550586189 | 7 | 193542 | 587 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | common bile duct disorder | pathwayBased | 0.102954655136439 | 16 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | common bile duct disorder | targetBased | 0.102954655136439 | 16 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | common bile duct disorder | targetBased | 0.102954655136439 | 16 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | common bile duct disorder | targetBased | 0.102954655136439 | 16 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | common bile duct disorder | targetBased | 0.102954655136439 | 16 | 124022 | 1156 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | common bile duct disorder | targetBased | 0.102714256663671 | 5 | 193542 | 587 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | bile duct disorder | targetBased | 0.174865308917946 | 22 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | bile duct disorder | targetBased | 0.174865308917946 | 22 | 296501 | 2737 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | bile duct disorder | pathwayBased | 0.20131006530027 | 22 | 376029 | 3978 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | bile duct disorder | targetBased | 0.137038067316657 | 7 | 336894 | 489 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | bile duct disorder | targetBased | 0.213737927874595 | 13 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | bile duct disorder | targetBased | 0.213737927874595 | 13 | 337446 | 1356 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | bile duct disorder | targetBased | 0.154814997950924 | 9 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | bile duct disorder | targetBased | 0.136225872415485 | 5 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | bile duct disorder | pathwayBased | 0.165148000435158 | 7 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | bile duct disorder | pathwayBased | 0.165148000435158 | 7 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | bile duct disorder | targetBased | 0.179948592537697 | 4 | 46715 | 2829 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | bile duct disorder | targetBased | 0.160115406202768 | 16 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | bile duct disorder | targetBased | 0.130204215901408 | 4 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | bile duct disorder | targetBased | 0.133004755719711 | 12 | 207811 | 220 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | bile duct disorder | targetBased | 0.167217702290312 | 6 | 47480 | 2010 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | bile duct disorder | pathwayBased | 0.23149539481692 | 258 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bile duct disorder | targetBased | 0.23149539481692 | 258 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bile duct disorder | targetBased | 0.23149539481692 | 258 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bile duct disorder | targetBased | 0.23149539481692 | 258 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | bile duct disorder | targetBased | 0.23149539481692 | 258 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | bile duct disorder | targetBased | 0.141311460105292 | 195 | 356517 | 1139 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | bile duct disorder | targetBased | 0.16887908609418 | 36 | 57013 | 201 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | bile duct disorder | targetBased | 0.121412241828512 | 5 | 343468 | 285 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | bile duct disorder | targetBased | 0.187341777733047 | 13 | 322361 | 619 |
| Luminescence Cell-Based Primary HTS to Identify Inhibitors of A1 Apoptosis. | BCL2L11_inhibitors | bile duct disorder | targetBased | 0.12089254391814 | 6 | 325630 | 216 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | bile duct disorder | targetBased | 0.178470306332188 | 11 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | bile duct disorder | pathwayBased | 0.178470306332188 | 11 | 403871 | 1243 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | bile duct disorder | targetBased | 0.127307347889318 | 10 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | bile duct disorder | targetBased | 0.127307347889318 | 10 | 218234 | 1620 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | bile duct disorder | targetBased | 0.139236852916198 | 6 | 344733 | 7043 |
| HTS of Smad transcription factor inhibitors | SMAD3 | bile duct disorder | targetBased | 0.201213887756849 | 7 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | bile duct disorder | targetBased | 0.174976088167904 | 30 | 193542 | 587 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | bile duct disorder | pathwayBased | 0.193756604488533 | 85 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | bile duct disorder | pathwayBased | 0.193756604488533 | 85 | 194666 | 1722 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | bile duct disorder | targetBased | 0.126366415720639 | 7 | 194628 | 267 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | bile duct disorder | targetBased | 0.162262749483003 | 4 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | bile duct disorder | targetBased | 0.162262749483003 | 4 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | bile duct disorder | targetBased | 0.109952188057476 | 8 | 195821 | 1625 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | bile duct disorder | targetBased | 0.140711123544983 | 4 | 347933 | 673 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | bile duct disorder | pathwayBased | 0.178767110927498 | 70 | 43989 | 342 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | bile duct disorder | targetBased | 0.23355036797594 | 18 | 71016 | 346 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | colonic disorder | pathwayBased | 0.171969034534272 | 19 | 376029 | 3978 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | colonic disorder | pathwayBased | 0.119078994116249 | 4 | 358556 | 3721 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | colonic disorder | targetBased | 0.206755728378382 | 20 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | colonic disorder | targetBased | 0.206755728378382 | 20 | 337446 | 1356 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | colonic disorder | targetBased | 0.154836661926854 | 8 | 194152 | 1342 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | colonic disorder | targetBased | 0.17564256735242 | 13 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | colonic disorder | pathwayBased | 0.137120646637068 | 21 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | colonic disorder | pathwayBased | 0.137120646637068 | 21 | 131324 | 544 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | colonic disorder | targetBased | 0.105332600461581 | 4 | 143816 | 859 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | colonic disorder | targetBased | 0.150027571590027 | 13 | 46715 | 2829 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | colonic disorder | targetBased | 0.150869237746611 | 39 | 57013 | 201 |
| Luminescence-based primary cell-based high throughput screening assay to identify activators of the Aryl Hydrocarbon Receptor (AHR) | AHR_activators | colonic disorder | targetBased | 0.118256571076123 | 206 | 324747 | 7988 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | colonic disorder | targetBased | 0.134060467132072 | 100 | 394050 | 3624 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | colonic disorder | targetBased | 0.148314488009712 | 12 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | colonic disorder | targetBased | 0.148314488009712 | 12 | 335239 | 695 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | colonic disorder | targetBased | 0.140052889942164 | 29 | 363840 | 2624 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | colonic disorder | targetBased | 0.173251015934994 | 11 | 363803 | 458 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | colonic disorder | targetBased | 0.136562113378297 | 5 | 369953 | 3123 |
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | colonic disorder | targetBased | 0.196694290124942 | 12 | 217959 | 771 |
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | colonic disorder | targetBased | 0.196694290124942 | 12 | 217959 | 569 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | colonic disorder | targetBased | 0.158193707487123 | 13 | 344733 | 7043 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | colonic disorder | targetBased | 0.141552184045967 | 4 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | colonic disorder | targetBased | 0.130889585483313 | 22 | 207811 | 220 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | colonic disorder | pathwayBased | 0.270593269175145 | 738 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | colonic disorder | targetBased | 0.270593269175145 | 738 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | colonic disorder | targetBased | 0.270593269175145 | 738 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | colonic disorder | targetBased | 0.270593269175145 | 738 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | colonic disorder | targetBased | 0.270593269175145 | 738 | 124022 | 1156 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | colonic disorder | pathwayBased | 0.134146780130385 | 31 | 7671 | 874 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | colonic disorder | targetBased | 0.134146780130385 | 31 | 304060 | 10600 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | colonic disorder | pathwayBased | 0.134146780130385 | 31 | 305439 | 16717 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | colonic disorder | targetBased | 0.239854198195393 | 32 | 322361 | 619 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | colonic disorder | targetBased | 0.136151997586347 | 8 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | colonic disorder | targetBased | 0.136151997586347 | 8 | 218234 | 1620 |
| qHTS for Inhibitors of WRN Helicase | WRN | colonic disorder | targetBased | 0.141868638271716 | 5 | 364011 | 1678 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | colonic disorder | targetBased | 0.210871674012731 | 127 | 193542 | 587 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | colonic disorder | targetBased | 0.171144866526617 | 6 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | colonic disorder | targetBased | 0.171144866526617 | 6 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | colonic disorder | targetBased | 0.171144866526617 | 6 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | colonic disorder | targetBased | 0.171144866526617 | 6 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | colonic disorder | targetBased | 0.171144866526617 | 6 | 359207 | 4555 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | colonic disorder | pathwayBased | 0.170398017381564 | 437 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | colonic disorder | pathwayBased | 0.170398017381564 | 437 | 194666 | 1722 |
| OTUD3 deubiquitinase inhibition: Primary qHTS | OTUD3 | colonic disorder | targetBased | 0.114461038153715 | 14 | 47480 | 334 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | colonic disorder | targetBased | 0.134962083210579 | 24 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | colonic disorder | targetBased | 0.120716228382883 | 25 | 218654 | 8241 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | colonic disorder | targetBased | 0.142164694272799 | 5 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | colonic disorder | targetBased | 0.163813569462516 | 7 | 331671 | 10022 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | colonic disorder | targetBased | 0.118726777754187 | 76 | 343468 | 734 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | colonic disorder | targetBased | 0.218518199680198 | 29 | 326888 | 645 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | colonic disorder | targetBased | 0.143955993792195 | 4 | 71016 | 346 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | colonic disorder | targetBased | 0.142529530078371 | 7 | 339297 | 1446 |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | colonic disorder | targetBased | 0.21417134494104 | 53 | 55710 | 315 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | intestinal motility disease | targetBased | 0.141409588541882 | 19 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | intestinal motility disease | targetBased | 0.141409588541882 | 19 | 331360 | 2760 |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | large intestine disorder | targetBased | 0.134288562643021 | 49 | 343786 | 1253 |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | large intestine disorder | targetBased | 0.134288562643021 | 49 | 296501 | 2737 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | large intestine disorder | pathwayBased | 0.228070992027438 | 174 | 376029 | 3978 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | large intestine disorder | pathwayBased | 0.137269222426945 | 7 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | large intestine disorder | targetBased | 0.152217052094048 | 40 | 336894 | 489 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | large intestine disorder | targetBased | 0.105595795953788 | 23 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | large intestine disorder | targetBased | 0.105595795953788 | 23 | 290726 | 2649 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | large intestine disorder | targetBased | 0.262908010888668 | 38 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | large intestine disorder | targetBased | 0.262908010888668 | 38 | 337446 | 1356 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | large intestine disorder | targetBased | 0.147064437995335 | 4 | 385746 | 932 |
| uHTS Identification of Diaphorase Inhibitors and Chemcical Oxidizers: Counter Screen for Diaphorase-based Primary Assays | DiaphoraseInhibitors | large intestine disorder | targetBased | 0.154836661926854 | 8 | 194152 | 1342 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | large intestine disorder | targetBased | 0.180886152989967 | 137 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | large intestine disorder | targetBased | 0.180886152989967 | 137 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | large intestine disorder | targetBased | 0.265429919371432 | 94 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | large intestine disorder | targetBased | 0.248782143633107 | 69 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | large intestine disorder | pathwayBased | 0.166657014789683 | 32 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | large intestine disorder | pathwayBased | 0.166657014789683 | 32 | 131324 | 544 |
| ROC1-CUL1 CTD Inhibitor di-Ub FRET Primary HTS Screen | RBX1 | large intestine disorder | targetBased | 0.127197447305982 | 9 | 143816 | 859 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | large intestine disorder | targetBased | 0.201690279516929 | 23 | 46715 | 2829 |
| Primary cell-based high throughput screening assay to identify inhibitors of kruppel-like factor 5 (KLF5) | KLF5 | large intestine disorder | targetBased | 0.162146041970988 | 19 | 290726 | 671 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | large intestine disorder | targetBased | 0.16133729345169 | 62 | 57013 | 201 |
| Luminescence-based primary cell-based high throughput screening assay to identify activators of the Aryl Hydrocarbon Receptor (AHR) | AHR_activators | large intestine disorder | targetBased | 0.118615639408968 | 214 | 324747 | 7988 |
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | large intestine disorder | targetBased | 0.185342811611515 | 7 | 564288 | 6030 |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify activators of the Protein Kinase A-R1A (PKA-R1A) complex | PRKAR1A | large intestine disorder | targetBased | 0.151250675861181 | 4 | 343468 | 285 |
| High Throughput Screen to Identify Compounds that increase expression of NF-kB in Human Neuronal Cells - Primary Screen | NFKB1 | large intestine disorder | pathwayBased | 0.168756070360562 | 1234 | 193265 | 3100 |
| uHTS identification of cystic fibrosis induced NFkb Inhibitors in a fluoresence assay | NFKB1 | large intestine disorder | pathwayBased | 0.168756070360562 | 1234 | 359244 | 3094 |
| Development of Small Molecule Probes of the Histone Methyltransferase, NSD2 Measured in Biochemical System Using Plate Reader - 7053-01_Inhibitor_SinglePoint_HTS_Activity_Set2 | NSD2 | large intestine disorder | targetBased | 0.179296826058977 | 4 | 309684 | 1662 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | large intestine disorder | targetBased | 0.106765490762238 | 35 | 338328 | 2501 |
| Toxoplasma gondii inhibition HTS in the presence of IFN-y | IFNG | large intestine disorder | targetBased | 0.148885738507163 | 891 | 67275 | 2509 |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | large intestine disorder | targetBased | 0.190608258230326 | 16 | 335239 | 991 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | large intestine disorder | targetBased | 0.190608258230326 | 16 | 335239 | 695 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | large intestine disorder | targetBased | 0.218647213853752 | 36 | 326888 | 645 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | large intestine disorder | targetBased | 0.18983241777785 | 45 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | large intestine disorder | targetBased | 0.176520859496463 | 68 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | large intestine disorder | pathwayBased | 0.176520859496463 | 68 | 403871 | 1243 |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of the liver receptor homolog-1 (LRH-1; NR5A2) | NR5A2 | large intestine disorder | targetBased | 0.173904853987899 | 14 | 363803 | 458 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | large intestine disorder | targetBased | 0.151722082053047 | 12 | 369953 | 3123 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | large intestine disorder | targetBased | 0.18095958189554 | 5 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | large intestine disorder | targetBased | 0.206512918988513 | 28 | 344733 | 7043 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | large intestine disorder | targetBased | 0.282586404960039 | 355 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | large intestine disorder | targetBased | 0.158167540689965 | 24 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | large intestine disorder | targetBased | 0.16441991437163 | 51 | 207811 | 220 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac activated mutant | RAC1 | large intestine disorder | targetBased | 0.158796742418323 | 5 | 194629 | 219 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Rac wildtype | RAC1 | large intestine disorder | targetBased | 0.158796742418323 | 5 | 194628 | 521 |
| High throughput screening of activators of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | large intestine disorder | targetBased | 0.148806690762262 | 4 | 305610 | 382 |
| High throughput screening of inhibitors of transient receptor potential cation channel C6 (TRPC6) | TRPC6 | large intestine disorder | targetBased | 0.148806690762262 | 4 | 305610 | 3253 |
| HTS for Estrogen Receptor-beta Coactivator Binding inhibitors | ESR2_inhibitors | large intestine disorder | targetBased | 0.106512728895391 | 66 | 86095 | 1114 |
| Luminescence-based cell-based primary high throughput screening assay to identify inhibitors of the Steroid Receptor Coactivator 2 (SRC2; NCOA2) | NCOA2 | large intestine disorder | targetBased | 0.147351816298633 | 4 | 368927 | 620 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | large intestine disorder | pathwayBased | 0.308826611252678 | 1700 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | large intestine disorder | targetBased | 0.308826611252678 | 1700 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | large intestine disorder | targetBased | 0.308826611252678 | 1700 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | large intestine disorder | targetBased | 0.308826611252678 | 1700 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | large intestine disorder | targetBased | 0.308826611252678 | 1700 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | large intestine disorder | targetBased | 0.252625920860032 | 436 | 356517 | 1139 |
| qHTS assay to identify small molecule antagonists of the retinoid-related orphan receptor gamma (ROR-gamma) signaling pathway | RORCgammaPathwayInhibitors | large intestine disorder | pathwayBased | 0.134171960841018 | 32 | 7671 | 874 |
| VP16 counterscreen qHTS for inhibitors of ROR gamma transcriptional activity | RORC | large intestine disorder | targetBased | 0.134171960841018 | 32 | 304060 | 10600 |
| qHTS for inhibitors of ROR gamma transcriptional activity | RORCgammaPathwayInhibitors | large intestine disorder | pathwayBased | 0.134171960841018 | 32 | 305439 | 16717 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | large intestine disorder | targetBased | 0.282794549839539 | 83 | 322361 | 619 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | large intestine disorder | targetBased | 0.19080672363384 | 7 | 335531 | 328 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | large intestine disorder | targetBased | 0.151260865961722 | 14 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | large intestine disorder | targetBased | 0.151260865961722 | 14 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | large intestine disorder | targetBased | 0.160579437976138 | 15 | 71016 | 346 |
| qHTS Assay for Inhibitors of HPGD (15-Hydroxyprostaglandin Dehydrogenase) | HPGD | large intestine disorder | targetBased | 0.156940032625911 | 32 | 148480 | 6428 |
| Absorbance-based primary biochemical high throughput screening assay to identify activators of procaspase-3 | procaspase3Activators | large intestine disorder | targetBased | 0.103830713271118 | 178 | 326024 | 350 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of HIV-1 LEDGF/p75 DNA Integration | PSIP1 | large intestine disorder | targetBased | 0.180924363966214 | 4 | 369953 | 2353 |
| qHTS for Inhibitors of WRN Helicase | WRN | large intestine disorder | targetBased | 0.242766866989144 | 14 | 364011 | 1678 |
| HTS of Smad transcription factor inhibitors | SMAD3 | large intestine disorder | targetBased | 0.274803506856109 | 63 | 88033 | 251 |
| uHTS luminescence assay for the identification of compounds that inhibit NOD2 | NOD2 | large intestine disorder | targetBased | 0.268452113239036 | 209 | 292323 | 1836 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | large intestine disorder | targetBased | 0.268417094971807 | 316 | 193542 | 587 |
| qHTS Assay for the Inhibitors of Human Flap endonuclease 1 (FEN1). | FEN1_inhibitors | large intestine disorder | targetBased | 0.102871363335938 | 9 | 386270 | 1331 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | large intestine disorder | targetBased | 0.171255692417983 | 7 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | large intestine disorder | targetBased | 0.171255692417983 | 7 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | large intestine disorder | targetBased | 0.171255692417983 | 7 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | large intestine disorder | targetBased | 0.171255692417983 | 7 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | large intestine disorder | targetBased | 0.171255692417983 | 7 | 359207 | 4555 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | large intestine disorder | pathwayBased | 0.180895637808501 | 538 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | large intestine disorder | pathwayBased | 0.180895637808501 | 538 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | large intestine disorder | targetBased | 0.164748862791827 | 10 | 339297 | 1446 |
| OTUD3 deubiquitinase inhibition: Primary qHTS | OTUD3 | large intestine disorder | targetBased | 0.114461038153715 | 14 | 47480 | 334 |
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | large intestine disorder | targetBased | 0.214172012932599 | 54 | 55710 | 315 |
| SMM ID4 Measured in Biochemical System Using Small Molecule MicroArray - 2128-01_Other_SinglePoint_HTS_Activity | ID4 | large intestine disorder | targetBased | 0.109491079300376 | 5 | 31324 | 362 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | large intestine disorder | targetBased | 0.192735364165936 | 50 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | large intestine disorder | targetBased | 0.144664582738475 | 28 | 218654 | 8241 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | large intestine disorder | targetBased | 0.146961132069313 | 4 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | large intestine disorder | targetBased | 0.146961132069313 | 4 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | large intestine disorder | targetBased | 0.13858808472361 | 22 | 195821 | 1625 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | large intestine disorder | targetBased | 0.206431590747704 | 10 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | large intestine disorder | targetBased | 0.176617717104892 | 9 | 331671 | 10022 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | large intestine disorder | pathwayBased | 0.243370349404526 | 204 | 43989 | 342 |
| Fluorescence-based cell-based primary high throughput screening assay to identify inhibitors of TLR9-MyD88 binding. | TLR9 | large intestine disorder | targetBased | 0.122298397346858 | 89 | 343468 | 734 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | small intestine disorder | pathwayBased | 0.146609163791844 | 9 | 376029 | 3978 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | small intestine disorder | targetBased | 0.110887129309367 | 10 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | small intestine disorder | targetBased | 0.110887129309367 | 10 | 290726 | 2649 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | small intestine disorder | targetBased | 0.13967346679307 | 11 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | small intestine disorder | targetBased | 0.13967346679307 | 11 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | small intestine disorder | targetBased | 0.153536662523345 | 6 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | small intestine disorder | targetBased | 0.25147180143239 | 110 | 359207 | 1432 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | small intestine disorder | targetBased | 0.153606520546873 | 5 | 57013 | 201 |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | small intestine disorder | targetBased | 0.183116719254692 | 6 | 326888 | 645 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | small intestine disorder | targetBased | 0.130490597561264 | 6 | 196177 | 519 |
| E3 Ligase HTS_1536 | MDM2 | small intestine disorder | targetBased | 0.105240693047513 | 15 | 207811 | 220 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | small intestine disorder | targetBased | 0.152156055151324 | 8 | 322361 | 619 |
| HTS of Smad transcription factor inhibitors | SMAD3 | small intestine disorder | targetBased | 0.168462445655091 | 5 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | small intestine disorder | targetBased | 0.154124167510169 | 26 | 193542 | 587 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | small intestine disorder | pathwayBased | 0.143732553309557 | 17 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | small intestine disorder | pathwayBased | 0.143732553309557 | 17 | 194666 | 1722 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | small intestine disorder | targetBased | 0.131497388941931 | 6 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | small intestine disorder | targetBased | 0.131497388941931 | 6 | 331360 | 2760 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | small intestine disorder | pathwayBased | 0.154410166348749 | 35 | 43989 | 342 |
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | large intestine disorder | targetBased | 0.150399635675161 | 127 | 394050 | 3624 |
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | large intestine disorder | targetBased | 0.196798431967435 | 13 | 217959 | 771 |
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | large intestine disorder | targetBased | 0.196798431967435 | 13 | 217959 | 569 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | large intestine disorder | targetBased | 0.14844873740403 | 4 | 47480 | 2010 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | small intestine disorder | targetBased | 0.162424730271162 | 6 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | small intestine disorder | targetBased | 0.162424730271162 | 6 | 337446 | 1356 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | small intestine disorder | targetBased | 0.181503795636043 | 17 | 263421 | 615 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | small intestine disorder | targetBased | 0.100474343332503 | 59 | 356517 | 1139 |
| qHTS Assay to Identify Small Molecule Activators of BRCA1 Expression | BRCA1 activation | upper digestive tract disorder | pathwayBased | 0.161486644810321 | 34 | 376029 | 3978 |
| HTS to identify compounds that promote myeloid differentiation with MLPCN compound set | HOXA9 | upper digestive tract disorder | pathwayBased | 0.102580658913278 | 6 | 358556 | 3721 |
| Fluorescence polarization to screen for inhibitors that disrupt the protein-protein interaction between Keap1 and Nrf2 Measured in Biochemical System Using Plate Reader - 2119-01_Inhibitor_SinglePoint_HTS_Activity | KEAP1 | upper digestive tract disorder | targetBased | 0.150321032541733 | 24 | 336894 | 489 |
| HTS for Tumor Hsp90 Inhibitors | HSP90 known inhibitor displacement | upper digestive tract disorder | targetBased | 0.116999052702556 | 17 | 63696 | 220 |
| Luminescence-based primary biochemical high throughput screening assay to identify inhibitors of the Heat Shock Protein 90 (HSP90) | HSP90AA1 | upper digestive tract disorder | targetBased | 0.116999052702556 | 17 | 290726 | 2649 |
| qHTS for Agonist of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | upper digestive tract disorder | targetBased | 0.145034469060499 | 4 | 334825 | 423 |
| qHTS for Antagonists of gsp, the Etiologic Mutation Responsible for Fibrous Dysplasia/McCune-Albright Syndrome: qHTS | GNAS | upper digestive tract disorder | targetBased | 0.145034469060499 | 4 | 337446 | 1356 |
| qHTS for Suppressors of FUS Proteinopathy: Primary screen using FUS-linked yeast assay | FUS_asupressors | upper digestive tract disorder | targetBased | 0.138614614299397 | 4 | 385746 | 932 |
| HTS of Estrogen Receptor- alpha Coactivator Binding inhibitors | ESR1_inhibitors | upper digestive tract disorder | targetBased | 0.206900623767966 | 52 | 86095 | 1442 |
| HTS of Estrogen Receptor- alpha Coactivator Binding Potentiators | ESR1_modulators | upper digestive tract disorder | targetBased | 0.206900623767966 | 52 | 86095 | 1151 |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | upper digestive tract disorder | targetBased | 0.165730732956527 | 31 | 217959 | 2390 |
| TRFRET-based biochemical primary high throughput screening assay to identify inhibitors of the interaction of the Ras and Rab interactor 1 protein (Rin1) and the c-abl oncogene 1, non-receptor tyrosine kinase (Abl) | ABL1_interaction | upper digestive tract disorder | targetBased | 0.167853003223802 | 11 | 359207 | 1432 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay | MAPK1 | upper digestive tract disorder | pathwayBased | 0.167328604663591 | 17 | 70898 | 707 |
| qHTS Assay for Inhibitors of the ERK Signaling Pathway using a Homogeneous Screening Assay; Stimulation with EGF | MAPK1 | upper digestive tract disorder | pathwayBased | 0.167328604663591 | 17 | 131324 | 544 |
| Dicer-mediated maturation of pre-microRNA | Dicer_inhibitors | upper digestive tract disorder | targetBased | 0.139952719795136 | 9 | 46715 | 2829 |
| EZH2/PRC2 methyltransferase inhibitors Measured in Biochemical System Using Plate Reader - 2125-01_Inhibitor_SinglePoint_HTS_Activity | EZH2_inhibitors | upper digestive tract disorder | targetBased | 0.156301703572436 | 50 | 57013 | 201 |
| Primary qHTS assay for inhibitors of human arachidonate 12S-lipoxygenase (ALOX12): Round 2 | ALOX12_inhibitors | upper digestive tract disorder | targetBased | 0.177237887811495 | 6 | 564288 | 6030 |
| uHTS identification of Gli-Sufu Antagonists in a luminescence reporter assay | GLI1 | upper digestive tract disorder | targetBased | 0.101711451740033 | 38 | 338328 | 2501 |
| uHTS identification of HIF-2a Inhibitors in a luminesence assay | HIF-2a_inhibitors | upper digestive tract disorder | targetBased | 0.168042672120834 | 9 | 363840 | 2624 |
| Nrf2 qHTS screen for inhibitors | nrf2Inhibitors | upper digestive tract disorder | targetBased | 0.219137549973724 | 58 | 360873 | 7438 |
| qHTS of Nrf2 Activators | Nrf2 activators | upper digestive tract disorder | pathwayBased | 0.219137549973724 | 58 | 403871 | 1243 |
| Fluorescence-based biochemical high throughput primary assay to identify inhibitors of phospholipase C isozymes (PLC-gamma1). | PLCG1 | upper digestive tract disorder | targetBased | 0.135230274597126 | 4 | 369953 | 3123 |
| qHTS Assay for Inhibitors of the Six1/Eya2 Interaction | SIX1 | upper digestive tract disorder | targetBased | 0.134387167760908 | 8 | 364053 | 2026 |
| Screen for inhibitors of the SWI/SNF chromatin remodeling complex (esBAF) in mouse embryonic stem cells with Luciferase reporter assay Measured in Cell-Based System Using Plate Reader - 2141-01_Inhibitor_SinglePoint_HTS_Activity | SMARCA4 | upper digestive tract disorder | targetBased | 0.216144931591686 | 19 | 344733 | 7043 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 99314 | 335 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 196176 | 782 |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 99314 | 390 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 196177 | 811 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 196176 | 670 |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | upper digestive tract disorder | targetBased | 0.137898774203478 | 35 | 196177 | 519 |
| qHTS Assay for Inhibitors Targeting the Menin-MLL Interaction in MLL Related Leukemias: Competition With Texas Red Labeled MLL-derived Mutant Peptide | Menin-MLL_inhibitors | upper digestive tract disorder | targetBased | 0.119075651192838 | 8 | 263421 | 615 |
| E3 Ligase HTS_1536 | MDM2 | upper digestive tract disorder | targetBased | 0.166792459560453 | 68 | 207811 | 220 |
| USP8 deubiquitinase inhibition: Primary qHTS | USP8 | upper digestive tract disorder | targetBased | 0.136683970652793 | 5 | 47480 | 2010 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | upper digestive tract disorder | pathwayBased | 0.286509685530083 | 976 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper digestive tract disorder | targetBased | 0.286509685530083 | 976 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper digestive tract disorder | targetBased | 0.286509685530083 | 976 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper digestive tract disorder | targetBased | 0.286509685530083 | 976 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | upper digestive tract disorder | targetBased | 0.286509685530083 | 976 | 124022 | 1156 |
| HTS Assay for Inhibitors of Akt Phophorylation: Primary Screen | AKT1_inhibitors | upper digestive tract disorder | targetBased | 0.14439203425888 | 387 | 356517 | 1139 |
| Inhibitors of CDC25B-CDK2/CyclinA interaction | Inhibitors of CDC25B-CDK2/CyclinA interaction | upper digestive tract disorder | targetBased | 0.112949418858417 | 31 | 93798 | 679 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 359518 | 300 |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 335652 | 1779 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 357537 | 806 |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 339887 | 1178 |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 362274 | 1056 |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | upper digestive tract disorder | targetBased | 0.21142960820566 | 7 | 336308 | 6862 |
| qHTS Assay for Identifying a Potential Treatment of Ataxia-Telangiectasia | ATM_modulators | upper digestive tract disorder | targetBased | 0.175103949253225 | 42 | 322361 | 619 |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | upper digestive tract disorder | targetBased | 0.173560529730411 | 4 | 335531 | 328 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | upper digestive tract disorder | targetBased | 0.153695536467353 | 15 | 215667 | 993 |
| uHTS identification of compounds inhibiting the binding between the RUNX1 Runt domain and CBFb-SMMHC via a fluorescence resonance energy transfer (FRET) assay. | RUNX1 | upper digestive tract disorder | targetBased | 0.153695536467353 | 15 | 218234 | 1620 |
| HTS for BAP1 Enzyme inhibitors | BAP1_inhibitors | upper digestive tract disorder | targetBased | 0.166506375767279 | 6 | 71016 | 346 |
| uHTS for 14-3-3/Bad interaction inhibitors | YWHAZ | upper digestive tract disorder | targetBased | 0.102570759914913 | 7 | 217332 | 1549 |
| qHTS for Inhibitors of WRN Helicase | WRN | upper digestive tract disorder | targetBased | 0.191754715622543 | 7 | 364011 | 1678 |
| HTS of Smad transcription factor inhibitors | SMAD3 | upper digestive tract disorder | targetBased | 0.194916238556379 | 22 | 88033 | 251 |
| High Throughput Imaging Assay for Beta-Catenin | betaCateninTranslocation | upper digestive tract disorder | targetBased | 0.192213291090885 | 53 | 193542 | 587 |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | ulcer disease | targetBased | 0.206158034285837 | 4 | 359207 | 1189 |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | ulcer disease | targetBased | 0.206158034285837 | 4 | 63676 | 1938 |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | ulcer disease | targetBased | 0.206158034285837 | 4 | 359207 | 316 |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | ulcer disease | targetBased | 0.206158034285837 | 4 | 63656 | 2179 |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | ulcer disease | targetBased | 0.206158034285837 | 4 | 359207 | 4555 |
| Primary cell-based high throughput screening assay to measure STAT3 activation | STAT3 | upper digestive tract disorder | pathwayBased | 0.162360550737552 | 170 | 194666 | 1772 |
| Primary cell-based high throughput screening assay to measure STAT3 inhibition | STAT3 | upper digestive tract disorder | pathwayBased | 0.162360550737552 | 170 | 194666 | 1722 |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | upper digestive tract disorder | targetBased | 0.191600561848195 | 5 | 339297 | 1446 |
| HTS to identify specific small molecule inhibitors of Ras and Ras-related GTPases specifically Ras wildtype | HRAS | upper digestive tract disorder | targetBased | 0.171768506342496 | 12 | 194628 | 267 |
| HTS for small molecule inhibitors of CHOP to regulate the unfolded protein response to ER stress | DDIT3_inhibitors | upper digestive tract disorder | targetBased | 0.135165226270177 | 7 | 218654 | 8241 |
| AlphaScreen-based biochemical high throughput primary assay to identify inhibitors of microphthalmia-associated transcription factor (MITF) | MITF inhibitors | upper digestive tract disorder | targetBased | 0.19148661159607 | 5 | 642362 | 5830 |
| MITF Measured in Cell-Based System Using Plate Reader - 2084-01_Inhibitor_SinglePoint_HTS_Activity | MITF | upper digestive tract disorder | targetBased | 0.19148661159607 | 5 | 331360 | 2760 |
| HCS assay for microtubule stabilizers | TUBB | upper digestive tract disorder | targetBased | 0.195319806594737 | 134 | 195821 | 1625 |
| qHTS Assay for Inhibitors of Bloom's syndrome helicase (BLM) | BLM_inhibitors | upper digestive tract disorder | targetBased | 0.187878760757735 | 7 | 347933 | 673 |
| uHTS for identification of Inhibitors of Mdm2/MdmX interaction in luminescent format. | MDM4 | upper digestive tract disorder | targetBased | 0.142872648633855 | 6 | 331671 | 10022 |
| Acumen qHTS Assay for Inhibitors of the mTORC1 Signaling Pathway in MEF (Tsc2-/-, p53-/-) Cells: Sytravon | MTOR | upper digestive tract disorder | pathwayBased | 0.198632349484476 | 156 | 43989 | 342 |
| qHTS assay for re-activators of p53 using a Luc reporter | TP53 | mouth mucosa disorder | pathwayBased | 0.108038132958635 | 7 | 321427 | 201 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth mucosa disorder | targetBased | 0.108038132958635 | 7 | 54509 | 528 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53 Null Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth mucosa disorder | targetBased | 0.108038132958635 | 7 | 54513 | 338 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Nonpermissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth mucosa disorder | targetBased | 0.108038132958635 | 7 | 125394 | 1890 |
| qHTS Screen for Compounds that Selectively Target Cancer Cells with p53 Mutations: Cytotoxicity of p53ts Cells at the Permissive Temperature | nonSmallCellLungCarcinomaWithP53Mutations | mouth mucosa disorder | targetBased | 0.108038132958635 | 7 | 124022 | 1156 |
| Primary cell-based high throughput screening assay to measure STAT1 activation | STAT1 activation | Genetic intestinal disease | pathwayBased | 0.139626497410024 | 5 | 195980 | 5134 |
| Primary cell-based high throughput screening assay to measure STAT1 inhibition | STAT1 | Genetic intestinal disease | targetBased | 0.139626497410024 | 5 | 195980 | 695 |
Some of these associations have also gone through clinical trials, as those in the graph below.
Find clinical trials details in table. Use scroll bar or search funtion to select specific drugs, molecular targets or diseases.
| BioAssay Name | program | gene | protName | diseaseName | molname | assayMode | clinicalPhase | clinicalStatus | studyStartDate | url | score | variantEffect | directionOnTrait | studyStopReason |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | anus disease | DILTIAZEM | targetBased | 2 | Not yet recruiting | 01/06/2021 | https://clinicaltrials.gov/study/NCT04887818 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | esophageal varices | PROPRANOLOL | targetBased | 4 | Unknown status | 01/06/2013 | https://clinicaltrials.gov/study/NCT02740166 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | esophageal varices | PROPRANOLOL | targetBased | 3 | Unknown status | https://clinicaltrials.gov/study/NCT01134692 | 0.7 | LoF | protect | ||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | dyspepsia | BUSPIRONE | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT03444831 | 1 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | dyspepsia | BUSPIRONE | targetBased | 4 | Completed | 01/03/2016 | https://clinicaltrials.gov/study/NCT03444831 | 1 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Dysphagia | BUSPIRONE | targetBased | 4 | Recruiting | 06/07/2021 | https://clinicaltrials.gov/study/NCT05629325 | 1 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Dysphagia | BUSPIRONE | targetBased | 4 | Recruiting | 06/07/2021 | https://clinicaltrials.gov/study/NCT05629325 | 1 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastroparesis | BUSPIRONE | targetBased | 2 | Completed | 27/08/2019 | https://clinicaltrials.gov/study/NCT03587142 | 0.2 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastroparesis | BUSPIRONE | targetBased | 2 | Completed | 27/08/2019 | https://clinicaltrials.gov/study/NCT03587142 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | gastroparesis | HALOPERIDOL | targetBased | 4 | Terminated | 01/11/2012 | https://clinicaltrials.gov/study/NCT02057549 | 1 | LoF | protect | PI left institution |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | stomatitis | MORPHINE | targetBased | 2 | Completed | 01/07/2011 | https://clinicaltrials.gov/study/NCT01837446 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | stomatitis | MORPHINE | targetBased | 2 | Completed | 01/07/2011 | https://clinicaltrials.gov/study/NCT01837446 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Completed | 01/12/2011 | https://clinicaltrials.gov/study/NCT01507246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Completed | 01/12/2011 | https://clinicaltrials.gov/study/NCT01507246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/12/2011 | https://clinicaltrials.gov/study/NCT02058290 | 1 | GoF | protect | Slower than expected enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/12/2011 | https://clinicaltrials.gov/study/NCT02058290 | 1 | GoF | protect | Slower than expected enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/05/2012 | https://clinicaltrials.gov/study/NCT01507233 | 1 | GoF | protect | Slow enrollment. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/05/2012 | https://clinicaltrials.gov/study/NCT01507233 | 1 | GoF | protect | Slow enrollment. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/03/2012 | https://clinicaltrials.gov/study/NCT01507220 | 1 | GoF | protect | Slow enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE | targetBased | 4 | Terminated | 01/03/2012 | https://clinicaltrials.gov/study/NCT01507220 | 1 | GoF | protect | Slow enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | CODEINE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | CODEINE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | gastrointestinal disease | CODEINE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastroesophageal reflux disease | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastroesophageal reflux disease | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastroesophageal reflux disease | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastroesophageal reflux disease | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastroesophageal reflux disease | PIRENZEPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A02BX03 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | NALOXONE | targetBased | 4 | Terminated | 20/09/2017 | https://clinicaltrials.gov/study/NCT03176316 | 1 | LoF | protect | The study was terminated by the Institutional Review Board. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | NALOXONE | targetBased | 4 | Terminated | 20/09/2017 | https://clinicaltrials.gov/study/NCT03176316 | 1 | LoF | protect | The study was terminated by the Institutional Review Board. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | ileus | NALOXONE | targetBased | 4 | Terminated | 20/09/2017 | https://clinicaltrials.gov/study/NCT03176316 | 1 | LoF | protect | The study was terminated by the Institutional Review Board. |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | irritable bowel syndrome | NALOXONE | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00108446 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | NALOXONE | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00108446 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | NALOXONE | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00108446 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | NALOXONE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | NALOXONE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | intestinal obstruction | NALOXONE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastric emptying | METOCLOPRAMIDE | targetBased | 4 | Completed | 14/12/2022 | https://clinicaltrials.gov/study/NCT05641051 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroschisis | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/03/2014 | https://clinicaltrials.gov/study/NCT02098915 | 0.7 | LoF | protect | Decision to prematurely close the trial was made because of poor recruitment. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 4 | Completed | 10/04/2021 | https://clinicaltrials.gov/study/NCT04771481 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 2 | Recruiting | 01/07/2020 | https://clinicaltrials.gov/study/NCT03840057 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Gastrointestinal hemorrhage | METOCLOPRAMIDE | targetBased | 3 | Recruiting | 16/03/2023 | https://clinicaltrials.gov/study/NCT06297954 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 27/03/2014 | https://clinicaltrials.gov/study/NCT02025725 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Completed | 15/04/2022 | https://clinicaltrials.gov/study/NCT05342818 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 2 | Terminated | 04/04/2014 | https://clinicaltrials.gov/study/NCT01934192 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE | targetBased | 3 | Completed | 01/04/2014 | https://clinicaltrials.gov/study/NCT02025751 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | METOCLOPRAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA01 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Not yet recruiting | 01/06/2024 | https://clinicaltrials.gov/study/NCT05876585 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | intestinal obstruction | METOCLOPRAMIDE | targetBased | 2 | Terminated | 26/06/2019 | https://clinicaltrials.gov/study/NCT04027348 | 0.2 | LoF | protect | low accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | METOCLOPRAMIDE | targetBased | 4 | Completed | 26/04/2017 | https://clinicaltrials.gov/study/NCT02907632 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 3 | Unknown status | 01/11/2015 | https://clinicaltrials.gov/study/NCT02619201 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Completed | 30/01/2024 | https://clinicaltrials.gov/study/NCT06390787 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE | targetBased | 4 | Completed | 01/11/2012 | https://clinicaltrials.gov/study/NCT02253524 | 1 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | gastrointestinal disease | PACLITAXEL | targetBased | 2 | Terminated | 01/01/1996 | https://clinicaltrials.gov/study/NCT00208936 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | salivary gland neoplasm | PACLITAXEL | targetBased | 2 | Recruiting | 10/12/2021 | https://clinicaltrials.gov/study/NCT05000892 | 0.2 | LoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | salivary gland neoplasm | PACLITAXEL | targetBased | 2 | Not yet recruiting | 30/04/2021 | https://clinicaltrials.gov/study/NCT04825938 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| HCS assay for microtubule stabilizers | TUBB | TUBB | Tubulin beta chain | aphthous ulcer | COLCHICINE | targetBased | 2 | Unknown status | 01/05/2007 | https://clinicaltrials.gov/study/NCT00723268 | 0.2 | LoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | diverticulitis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/11/2016 | https://clinicaltrials.gov/study/NCT02785549 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | gastrointestinal disease | ACETAMINOPHEN | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | intestinal obstruction | ACETAMINOPHEN | targetBased | 4 | Enrolling by invitation | 11/10/2023 | https://clinicaltrials.gov/study/NCT05878015 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | gastrointestinal mucositis | ACETAMINOPHEN | targetBased | 4 | Completed | 01/02/2012 | https://clinicaltrials.gov/study/NCT01822665 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Vomiting | ACETAMINOPHEN | targetBased | 3 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02462811 | 0.7 | protect | ||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | FENTANYL | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00108446 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | FENTANYL | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00108446 | 0.2 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | ALFENTANIL | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01199458 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | ALFENTANIL | targetBased | 4 | Completed | 01/09/2010 | https://clinicaltrials.gov/study/NCT01199458 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | dyspepsia | MIRTAZAPINE | targetBased | 4 | Completed | 01/09/2006 | https://clinicaltrials.gov/study/NCT01240096 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Gastrointestinal hemorrhage | EPINEPHRINE | targetBased | 3 | Completed | 07/09/2015 | https://clinicaltrials.gov/study/NCT02534571 | 0.7 | GoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | Gastrointestinal hemorrhage | EPINEPHRINE | targetBased | 4 | Unknown status | 01/09/2015 | https://clinicaltrials.gov/study/NCT02537353 | 1 | GoF | protect | |
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | CACNA1H | Voltage-dependent T-type calcium channel subunit alpha-1H | irritable bowel syndrome | ETHOSUXIMIDE | targetBased | 2 | Unknown status | 30/04/2018 | https://clinicaltrials.gov/study/NCT02973542 | 0.2 | LoF | protect | |
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | CACNA1H | Voltage-dependent T-type calcium channel subunit alpha-1H | irritable bowel syndrome | ETHOSUXIMIDE | targetBased | 3 | Recruiting | 01/11/2019 | https://clinicaltrials.gov/study/NCT04217733 | 0.7 | LoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | eosinophilic esophagitis | MESALAMINE | targetBased | 2 | Completed | 09/02/2023 | https://clinicaltrials.gov/study/NCT05488405 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 3 | Completed | 01/10/2005 | https://clinicaltrials.gov/study/NCT01120340 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticular disease | MESALAMINE | targetBased | 4 | Completed | 01/01/2009 | https://clinicaltrials.gov/study/NCT01534754 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | gastrointestinal disease | MESALAMINE | targetBased | 2 | Unknown status | 01/01/2014 | https://clinicaltrials.gov/study/NCT02044952 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/07/2010 | https://clinicaltrials.gov/study/NCT01177410 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/06/2010 | https://clinicaltrials.gov/study/NCT01412372 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 3 | Completed | 01/12/2007 | https://clinicaltrials.gov/study/NCT00626288 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Completed | 01/03/2011 | https://clinicaltrials.gov/study/NCT01316718 | 1 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01327300 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/04/2012 | https://clinicaltrials.gov/study/NCT01699438 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00774007 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | irritable bowel syndrome | MESALAMINE | targetBased | 4 | Withdrawn | 01/09/2014 | https://clinicaltrials.gov/study/NCT02190526 | 1 | GoF | protect | Study stopped due to lack of volunteer patients. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2008 | https://clinicaltrials.gov/study/NCT00695643 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Terminated | 01/01/2010 | https://clinicaltrials.gov/study/NCT01038739 | 0.35 | GoF | protect | Stopped due to futility. |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 2 | Completed | 01/11/2007 | https://clinicaltrials.gov/study/NCT00554099 | 0.2 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Measurement of TR-FRET detection format artefact in the screen for agonists of steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 1 (SRC-1) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 2 (SRC-2) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Primary biochemical High Throughput Screening assay for agonists of the steroid receptor coactivator 3 (SRC-3) recruitment by the peroxisome proliferator-activated receptor gamma (PPARgamma) | PPARG | PPARG | Peroxisome proliferator-activated receptor gamma | diverticulitis | MESALAMINE | targetBased | 3 | Completed | 06/12/2007 | https://clinicaltrials.gov/study/NCT00545103 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 10/08/2017 | https://clinicaltrials.gov/study/NCT03118986 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Recruiting | 01/09/2018 | https://clinicaltrials.gov/study/NCT03679182 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | Vomiting | OLANZAPINE | targetBased | 2 | Terminated | 01/07/2010 | https://clinicaltrials.gov/study/NCT01148264 | 0.2 | LoF | protect | poor enrolment |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | esophageal varices | CARVEDILOL | targetBased | 4 | Unknown status | 01/12/2015 | https://clinicaltrials.gov/study/NCT02638415 | 1 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | esophageal varices | CARVEDILOL | targetBased | 2 | Recruiting | 01/10/2018 | https://clinicaltrials.gov/study/NCT04499898 | 0.2 | LoF | protect | |
| HTS for Beta-2AR agonists via FAP method | ADRB2_activators | ADRB2 | Beta-2 adrenergic receptor | esophageal varices | CARVEDILOL | targetBased | 4 | Recruiting | 17/06/2019 | https://clinicaltrials.gov/study/NCT03776955 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ALIZAPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA05 | 0.7 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae8237f0-95a6-4163-b476-25c0509ef151 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae8237f0-95a6-4163-b476-25c0509ef151 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be99224e-dd49-4628-a5bf-ed8469ffd4a4 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be99224e-dd49-4628-a5bf-ed8469ffd4a4 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7346bd7e-8af0-4de3-a6f8-0769b7a3135c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7346bd7e-8af0-4de3-a6f8-0769b7a3135c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a66d2f97-8d22-482d-b18b-f58f1d882f42 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a66d2f97-8d22-482d-b18b-f58f1d882f42 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5efdfc41-a7a4-43cf-858a-7da8ea1399ad | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5efdfc41-a7a4-43cf-858a-7da8ea1399ad | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c97152f0-73ae-4463-8177-8f9250d84252 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c97152f0-73ae-4463-8177-8f9250d84252 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT02340481 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT02340481 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1985ce5e-1209-495e-94c9-03d6e2832627 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1985ce5e-1209-495e-94c9-03d6e2832627 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d358a9c1-47fa-424c-8cc0-24010cfb7ee3 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d358a9c1-47fa-424c-8cc0-24010cfb7ee3 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=146e0229-1444-4ad8-8d31-1b02c4222df0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=146e0229-1444-4ad8-8d31-1b02c4222df0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00685607 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/10/2008 | https://clinicaltrials.gov/study/NCT00685607 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6deb0658-c96e-40bd-b3a3-9655a4769270 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6deb0658-c96e-40bd-b3a3-9655a4769270 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d98f5f8-abab-4f30-90ad-19e030de2a76 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d98f5f8-abab-4f30-90ad-19e030de2a76 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c4a072e-88e6-4c35-adf2-fe81f3bcf4c9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c4a072e-88e6-4c35-adf2-fe81f3bcf4c9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35df0cb2-ca82-49ee-afad-4431db5708d6 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35df0cb2-ca82-49ee-afad-4431db5708d6 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72a7ae47-cdf3-4949-b9f8-f29b153f787f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72a7ae47-cdf3-4949-b9f8-f29b153f787f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf47ac3-746f-47c3-82b2-32ac979ff963 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf47ac3-746f-47c3-82b2-32ac979ff963 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fdf0f19-a8d3-408c-adcb-dcaa0a4c2741 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fdf0f19-a8d3-408c-adcb-dcaa0a4c2741 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d23f73b-afc2-448d-afb3-b167d02cfc06 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d23f73b-afc2-448d-afb3-b167d02cfc06 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9b96a5d-2754-4958-b066-c2e65f71dbb0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9b96a5d-2754-4958-b066-c2e65f71dbb0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01434d38-2c9d-4222-adb8-cf7b4909cac8 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01434d38-2c9d-4222-adb8-cf7b4909cac8 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c6a3322d-943c-4f7a-9975-94d3fa38b12c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c6a3322d-943c-4f7a-9975-94d3fa38b12c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad71d4ab-d293-47a2-8f3f-2c9f42d76e40 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad71d4ab-d293-47a2-8f3f-2c9f42d76e40 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc639a62-8bc9-44bd-a07f-639079e30331 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc639a62-8bc9-44bd-a07f-639079e30331 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Completed | 26/03/1997 | https://clinicaltrials.gov/study/NCT00003057 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Completed | 26/03/1997 | https://clinicaltrials.gov/study/NCT00003057 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2038ce27-2b16-44be-8d57-ebd6dbaebf26 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2038ce27-2b16-44be-8d57-ebd6dbaebf26 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00292344 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00292344 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3310a3c2-389a-4b27-a478-67c905789854 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3310a3c2-389a-4b27-a478-67c905789854 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b43dded-bf30-4967-b78f-8111a2f28979 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b43dded-bf30-4967-b78f-8111a2f28979 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=853f76d1-eb22-4fad-8d72-46d11b538f89 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=853f76d1-eb22-4fad-8d72-46d11b538f89 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Terminated | 01/01/2003 | https://clinicaltrials.gov/study/NCT00650637 | 0.7 | GoF | protect | The study was prematurely discontinued due to administrative reasons on August 18, 2003. There were no safety concerns that led to the decision to terminate. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 3 | Terminated | 01/01/2003 | https://clinicaltrials.gov/study/NCT00650637 | 0.7 | GoF | protect | The study was prematurely discontinued due to administrative reasons on August 18, 2003. There were no safety concerns that led to the decision to terminate. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e0fc3-334f-4c7d-a661-486efd030717 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e0fc3-334f-4c7d-a661-486efd030717 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c2f4b11-6c45-4270-9b0b-ce73d5eae65f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c2f4b11-6c45-4270-9b0b-ce73d5eae65f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03a1429b-cd24-4cb7-84bb-5ec7a7b2b687 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03a1429b-cd24-4cb7-84bb-5ec7a7b2b687 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00359970 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/06/2002 | https://clinicaltrials.gov/study/NCT00359970 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00807326 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00807326 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=580e6cd4-3f05-4996-9dc3-c50d757ae210 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=580e6cd4-3f05-4996-9dc3-c50d757ae210 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=39deeb78-f9c3-421e-8455-ff5ab6820718 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=39deeb78-f9c3-421e-8455-ff5ab6820718 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e5f53-e587-411b-a0ec-b32452ec0d21 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e5f53-e587-411b-a0ec-b32452ec0d21 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6615a259-98ee-4d07-adc0-8d7f490e19f7 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6615a259-98ee-4d07-adc0-8d7f490e19f7 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a64b9fed-bf27-4e02-876e-72a628a0d8ef | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a64b9fed-bf27-4e02-876e-72a628a0d8ef | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=37a300a8-8c4e-46ff-bbff-857384c4a640 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=37a300a8-8c4e-46ff-bbff-857384c4a640 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9dd0d20c-7c02-47d9-b20e-412b0e5dfe8f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9dd0d20c-7c02-47d9-b20e-412b0e5dfe8f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8143434f-070b-43ea-8af9-30d142765b18 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8143434f-070b-43ea-8af9-30d142765b18 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab07bfd1-d2d3-427a-866d-89a10d40a4e9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab07bfd1-d2d3-427a-866d-89a10d40a4e9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 3 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT02008565 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 3 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT02008565 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 2 | Completed | 01/07/2008 | https://clinicaltrials.gov/study/NCT00727649 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 2 | Completed | 01/07/2008 | https://clinicaltrials.gov/study/NCT00727649 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 4 | Withdrawn | 01/10/2009 | https://clinicaltrials.gov/study/NCT00933465 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE | targetBased | 4 | Withdrawn | 01/10/2009 | https://clinicaltrials.gov/study/NCT00933465 | 1 | GoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | burning mouth syndrome | CAPSAICIN | targetBased | 4 | Recruiting | 01/11/2023 | https://clinicaltrials.gov/study/NCT06040190 | 1 | protect | ||
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | Vomiting | CAPSAICIN | targetBased | 2 | Completed | 20/12/2017 | https://clinicaltrials.gov/study/NCT03223350 | 0.2 | protect | ||
| Inhibitors of the vitamin D receptor (VDR): qHTS | VDR | VDR | Vitamin D3 receptor | irritable bowel syndrome | CHOLECALCIFEROL | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02026518 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of the mouse 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) | HTR2A | HTR2A | 5-hydroxytryptamine receptor 2A | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | Vomiting | DROPERIDOL | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT00702442 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DICYCLOMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA07 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DICYCLOMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA07 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DICYCLOMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA07 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DICYCLOMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA07 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DICYCLOMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA07 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | CLEBOPRIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA06 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE BROMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE BROMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE BROMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE BROMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE BROMIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| High Throughput Screening for Cocaine Antagonists: Primary Screen | SLC6A3 | SLC6A3 | Sodium-dependent dopamine transporter | Dysphagia | MODAFINIL | targetBased | 2 | Completed | 01/03/2010 | https://clinicaltrials.gov/study/NCT01085903 | 0.2 | LoF | protect | |
| HTS Assay for Compounds that Act as Agonists of the Vanilloid Receptor 1 | TRPV1 | TRPV1 | Transient receptor potential cation channel subfamily V member 1 | irritable bowel syndrome | SB-705498 | targetBased | 2 | Terminated | 26/01/2007 | https://clinicaltrials.gov/study/NCT00461682 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 4 | Unknown status | 01/10/2014 | https://clinicaltrials.gov/study/NCT02264587 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Recruiting | 27/03/2023 | https://clinicaltrials.gov/study/NCT05832151 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/03/2010 | https://clinicaltrials.gov/study/NCT01378884 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 01/10/2008 | https://clinicaltrials.gov/study/NCT00760461 | 0.2 | LoF | protect | Enrolled subjects were unable to receive drug from dispensing pharmacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Enrolling by invitation | 01/05/2015 | https://clinicaltrials.gov/study/NCT02757534 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Terminated | 17/02/2020 | https://clinicaltrials.gov/study/NCT04208698 | 0.2 | LoF | protect | Trial was terminated due to the impact of COVID-19 on trial activities. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | DOMPERIDONE | targetBased | 2 | Completed | 11/09/2019 | https://clinicaltrials.gov/study/NCT04026997 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | DOMPERIDONE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03FA03 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | DOMPERIDONE | targetBased | 3 | Terminated | 07/12/2015 | https://clinicaltrials.gov/study/NCT02699385 | 0.35 | LoF | protect | Study prematurely terminated upon recommendation of IDMC due to lack of efficacy. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 26/06/2019 | https://clinicaltrials.gov/study/NCT05030116 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 4 | Completed | 27/08/2018 | https://clinicaltrials.gov/study/NCT03617016 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | DOMPERIDONE | targetBased | 3 | Completed | 01/10/2010 | https://clinicaltrials.gov/study/NCT01355276 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 3 | Withdrawn | 01/08/2013 | https://clinicaltrials.gov/study/NCT01710462 | 0.7 | LoF | protect | Change of company's strategy |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Withdrawn | 01/03/2018 | https://clinicaltrials.gov/study/NCT03355170 | 1 | LoF | protect | The sponsor decided to continue with a different design and a different protocol. |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Completed | 01/01/2014 | https://clinicaltrials.gov/study/NCT02958046 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroesophageal reflux disease | DOMPERIDONE | targetBased | 4 | Unknown status | 01/12/2013 | https://clinicaltrials.gov/study/NCT02140073 | 1 | LoF | protect | |
| Primary cell-based high throughput assay for inhibitors of the Janus kinase 2 mutant JAK2V617F | JAK2 | JAK2 | Tyrosine-protein kinase , Tyrosine-protein kinase JAK2 | pouchitis | TOFACITINIB | targetBased | 2 | Terminated | 25/01/2021 | https://clinicaltrials.gov/study/NCT04580277 | 0.2 | LoF | protect | low recruitment |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | Vomiting | HYDROCODONE | targetBased | 3 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02462811 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Vomiting | HYDROCODONE | targetBased | 3 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02462811 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Vomiting | HYDROCODONE | targetBased | 3 | Completed | 01/09/2014 | https://clinicaltrials.gov/study/NCT02462811 | 0.7 | GoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | EXENATIDE | targetBased | 4 | Withdrawn | 01/03/2013 | https://clinicaltrials.gov/study/NCT01818648 | 1 | GoF | protect | Study was withdrawn by PI due to decision to study a different medication. |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | EXENATIDE | targetBased | 4 | Withdrawn | 01/03/2013 | https://clinicaltrials.gov/study/NCT01818648 | 1 | GoF | protect | Study was withdrawn by PI due to decision to study a different medication. |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | EXENATIDE | targetBased | 4 | Withdrawn | 01/03/2013 | https://clinicaltrials.gov/study/NCT01818648 | 1 | GoF | protect | Study was withdrawn by PI due to decision to study a different medication. |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastrointestinal disease | ALVERINE | targetBased | 2 | https://www.whocc.no/atc_ddd_index/?code=A03AX58 | 0.2 | LoF | protect | |||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastrointestinal disease | ALVERINE | targetBased | 2 | https://www.whocc.no/atc_ddd_index/?code=A03AX58 | 0.2 | LoF | protect | |||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastrointestinal disease | ALVERINE | targetBased | 2 | https://www.whocc.no/atc_ddd_index/?code=A03AX08 | 0.2 | LoF | protect | |||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | gastrointestinal disease | ALVERINE | targetBased | 2 | https://www.whocc.no/atc_ddd_index/?code=A03AX08 | 0.2 | LoF | protect | |||
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT00542295 | 1 | LoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 01/07/2007 | https://clinicaltrials.gov/study/NCT00542295 | 1 | LoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 03/09/2018 | https://clinicaltrials.gov/study/NCT04145856 | 1 | LoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 03/09/2018 | https://clinicaltrials.gov/study/NCT04145856 | 1 | LoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 01/12/2009 | https://clinicaltrials.gov/study/NCT01404923 | 1 | LoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | irritable bowel syndrome | ALVERINE | targetBased | 4 | Completed | 01/12/2009 | https://clinicaltrials.gov/study/NCT01404923 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | BROMOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA04 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | BROMOPRIDE | targetBased | 3 | Completed | 17/01/2017 | https://clinicaltrials.gov/study/NCT02604576 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENCYCLIMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA01 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENCYCLIMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA01 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENCYCLIMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA01 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENCYCLIMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA01 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENCYCLIMINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AA01 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | HYDROMORPHONE | targetBased | 3 | Completed | 01/04/2005 | https://clinicaltrials.gov/study/NCT00600158 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | HYDROMORPHONE | targetBased | 3 | Completed | 01/04/2005 | https://clinicaltrials.gov/study/NCT00600158 | 0.7 | GoF | protect | |
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | anus disease | NIFEDIPINE | targetBased | 2 | Not yet recruiting | 01/06/2021 | https://clinicaltrials.gov/study/NCT04887818 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00205842 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/06/2004 | https://clinicaltrials.gov/study/NCT00205842 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00388479 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/12/2001 | https://clinicaltrials.gov/study/NCT00388479 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Terminated | 01/09/2017 | https://clinicaltrials.gov/study/NCT03068975 | 1 | LoF | protect | This study was terminated due to failure to enroll, a low volume of patients met criteria. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Terminated | 01/09/2017 | https://clinicaltrials.gov/study/NCT03068975 | 1 | LoF | protect | This study was terminated due to failure to enroll, a low volume of patients met criteria. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Recruiting | 01/08/2020 | https://clinicaltrials.gov/study/NCT04405037 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Recruiting | 01/08/2020 | https://clinicaltrials.gov/study/NCT04405037 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Terminated | 02/12/2015 | https://clinicaltrials.gov/study/NCT02742181 | 0.7 | LoF | protect | terminated due to lack of enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Terminated | 02/12/2015 | https://clinicaltrials.gov/study/NCT02742181 | 0.7 | LoF | protect | terminated due to lack of enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/01/2002 | https://clinicaltrials.gov/study/NCT00388401 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/01/2002 | https://clinicaltrials.gov/study/NCT00388401 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00708201 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 4 | Completed | 01/03/2009 | https://clinicaltrials.gov/study/NCT00708201 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/03/2001 | https://clinicaltrials.gov/study/NCT00388258 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 3 | Completed | 01/03/2001 | https://clinicaltrials.gov/study/NCT00388258 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 2 | Terminated | 08/03/2018 | https://clinicaltrials.gov/study/NCT03352414 | 0.2 | LoF | protect | stopped by sponsor |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | ALVIMOPAN | targetBased | 2 | Terminated | 08/03/2018 | https://clinicaltrials.gov/study/NCT03352414 | 0.2 | LoF | protect | stopped by sponsor |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00259922 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00259922 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00101998 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 2 | Completed | 01/10/2003 | https://clinicaltrials.gov/study/NCT00101998 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00135577 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 2 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00135577 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00241722 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00241722 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00256932 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | ALVIMOPAN | targetBased | 3 | Completed | 01/08/2005 | https://clinicaltrials.gov/study/NCT00256932 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ATROPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03BA01 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ATROPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03BA01 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ATROPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03BA01 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ATROPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03BA01 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ATROPINE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03BA01 | 1 | LoF | protect | |||
| Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer (HTRF) Assay | CACNA1B_modulators | CACNA1B | Voltage-dependent N-type calcium channel subunit alpha-1D | gastrointestinal disease | PHLOROGLUCINOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AX12 | 1 | LoF | protect | |||
| Inhibitors of Cav3 T-type Calcium Channels: Primary Screen | CACNA1H_inhibitors | CACNA1H | Voltage-dependent T-type calcium channel subunit alpha-1H | gastrointestinal disease | PHLOROGLUCINOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AX12 | 1 | LoF | protect | |||
| HTS for Inhibition of CaV1.3 ICDI/IQ interaction using a live-cell FRET assay Measured in Cell-Based System Using Plate Reader - 7081-01_Inhibitor_SinglePoint_HTS_Activity | CACNA1D_inhibitors | CACNA1D | Voltage-dependent N-type calcium channel subunit alpha-1B | gastrointestinal disease | PHLOROGLUCINOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AX12 | 1 | LoF | protect | |||
| Modulation of AMPAR-stargazin complexes | AMPAStargazinComplexModulators | CACNG2 | Voltage-dependent calcium channel gamma-2 subunit | gastrointestinal disease | PHLOROGLUCINOL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AX12 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | MEPENZOLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB12 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | MEPENZOLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB12 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | MEPENZOLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB12 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | MEPENZOLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB12 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | MEPENZOLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB12 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00807326 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/11/2008 | https://clinicaltrials.gov/study/NCT00807326 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6deb0658-c96e-40bd-b3a3-9655a4769270 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6deb0658-c96e-40bd-b3a3-9655a4769270 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT02340481 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 3 | Completed | 01/07/2005 | https://clinicaltrials.gov/study/NCT02340481 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6615a259-98ee-4d07-adc0-8d7f490e19f7 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6615a259-98ee-4d07-adc0-8d7f490e19f7 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9dd0d20c-7c02-47d9-b20e-412b0e5dfe8f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9dd0d20c-7c02-47d9-b20e-412b0e5dfe8f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5efdfc41-a7a4-43cf-858a-7da8ea1399ad | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5efdfc41-a7a4-43cf-858a-7da8ea1399ad | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b43dded-bf30-4967-b78f-8111a2f28979 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b43dded-bf30-4967-b78f-8111a2f28979 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d98f5f8-abab-4f30-90ad-19e030de2a76 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5d98f5f8-abab-4f30-90ad-19e030de2a76 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e5f53-e587-411b-a0ec-b32452ec0d21 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e5f53-e587-411b-a0ec-b32452ec0d21 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 3 | Completed | 26/03/1997 | https://clinicaltrials.gov/study/NCT00003057 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 3 | Completed | 26/03/1997 | https://clinicaltrials.gov/study/NCT00003057 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8143434f-070b-43ea-8af9-30d142765b18 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8143434f-070b-43ea-8af9-30d142765b18 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2038ce27-2b16-44be-8d57-ebd6dbaebf26 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2038ce27-2b16-44be-8d57-ebd6dbaebf26 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c6a3322d-943c-4f7a-9975-94d3fa38b12c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c6a3322d-943c-4f7a-9975-94d3fa38b12c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab07bfd1-d2d3-427a-866d-89a10d40a4e9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ab07bfd1-d2d3-427a-866d-89a10d40a4e9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72a7ae47-cdf3-4949-b9f8-f29b153f787f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=72a7ae47-cdf3-4949-b9f8-f29b153f787f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9b96a5d-2754-4958-b066-c2e65f71dbb0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f9b96a5d-2754-4958-b066-c2e65f71dbb0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c97152f0-73ae-4463-8177-8f9250d84252 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c97152f0-73ae-4463-8177-8f9250d84252 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=39deeb78-f9c3-421e-8455-ff5ab6820718 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=39deeb78-f9c3-421e-8455-ff5ab6820718 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be99224e-dd49-4628-a5bf-ed8469ffd4a4 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=be99224e-dd49-4628-a5bf-ed8469ffd4a4 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c2f4b11-6c45-4270-9b0b-ce73d5eae65f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c2f4b11-6c45-4270-9b0b-ce73d5eae65f | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=146e0229-1444-4ad8-8d31-1b02c4222df0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=146e0229-1444-4ad8-8d31-1b02c4222df0 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3310a3c2-389a-4b27-a478-67c905789854 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3310a3c2-389a-4b27-a478-67c905789854 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a64b9fed-bf27-4e02-876e-72a628a0d8ef | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a64b9fed-bf27-4e02-876e-72a628a0d8ef | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03a1429b-cd24-4cb7-84bb-5ec7a7b2b687 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=03a1429b-cd24-4cb7-84bb-5ec7a7b2b687 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a66d2f97-8d22-482d-b18b-f58f1d882f42 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a66d2f97-8d22-482d-b18b-f58f1d882f42 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e0fc3-334f-4c7d-a661-486efd030717 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3f7e0fc3-334f-4c7d-a661-486efd030717 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf47ac3-746f-47c3-82b2-32ac979ff963 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf47ac3-746f-47c3-82b2-32ac979ff963 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad71d4ab-d293-47a2-8f3f-2c9f42d76e40 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ad71d4ab-d293-47a2-8f3f-2c9f42d76e40 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=37a300a8-8c4e-46ff-bbff-857384c4a640 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=37a300a8-8c4e-46ff-bbff-857384c4a640 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=853f76d1-eb22-4fad-8d72-46d11b538f89 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=853f76d1-eb22-4fad-8d72-46d11b538f89 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d23f73b-afc2-448d-afb3-b167d02cfc06 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3d23f73b-afc2-448d-afb3-b167d02cfc06 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=580e6cd4-3f05-4996-9dc3-c50d757ae210 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=580e6cd4-3f05-4996-9dc3-c50d757ae210 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c4a072e-88e6-4c35-adf2-fe81f3bcf4c9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c4a072e-88e6-4c35-adf2-fe81f3bcf4c9 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35df0cb2-ca82-49ee-afad-4431db5708d6 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=35df0cb2-ca82-49ee-afad-4431db5708d6 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1985ce5e-1209-495e-94c9-03d6e2832627 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1985ce5e-1209-495e-94c9-03d6e2832627 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fdf0f19-a8d3-408c-adcb-dcaa0a4c2741 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5fdf0f19-a8d3-408c-adcb-dcaa0a4c2741 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01434d38-2c9d-4222-adb8-cf7b4909cac8 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01434d38-2c9d-4222-adb8-cf7b4909cac8 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d358a9c1-47fa-424c-8cc0-24010cfb7ee3 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d358a9c1-47fa-424c-8cc0-24010cfb7ee3 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7346bd7e-8af0-4de3-a6f8-0769b7a3135c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7346bd7e-8af0-4de3-a6f8-0769b7a3135c | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae8237f0-95a6-4163-b476-25c0509ef151 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae8237f0-95a6-4163-b476-25c0509ef151 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc639a62-8bc9-44bd-a07f-639079e30331 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | Diarrhea | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cc639a62-8bc9-44bd-a07f-639079e30331 | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/10/2009 | https://clinicaltrials.gov/study/NCT00933465 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | fecal incontinence | LOPERAMIDE HYDROCHLORIDE | targetBased | 4 | Withdrawn | 01/10/2009 | https://clinicaltrials.gov/study/NCT00933465 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | NALOXONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | NALOXONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | intestinal obstruction | NALOXONE HYDROCHLORIDE | targetBased | 3 | Unknown status | 01/05/2000 | https://clinicaltrials.gov/study/NCT00020605 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | intestinal disease | SCOPOLAMINE | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01929044 | 0.7 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | intestinal disease | SCOPOLAMINE | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01929044 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | intestinal disease | SCOPOLAMINE | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01929044 | 0.7 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | intestinal disease | SCOPOLAMINE | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01929044 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | intestinal disease | SCOPOLAMINE | targetBased | 3 | Completed | 01/08/2013 | https://clinicaltrials.gov/study/NCT01929044 | 0.7 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | SCOPOLAMINE | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | SCOPOLAMINE | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | SCOPOLAMINE | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | SCOPOLAMINE | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | SCOPOLAMINE | targetBased | 2 | Completed | 01/02/1998 | https://clinicaltrials.gov/study/NCT02229786 | 0.2 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Vomiting | SCOPOLAMINE | targetBased | 4 | Completed | 01/10/2021 | https://clinicaltrials.gov/study/NCT04785118 | 1 | LoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Vomiting | SCOPOLAMINE | targetBased | 4 | Completed | 01/10/2021 | https://clinicaltrials.gov/study/NCT04785118 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Vomiting | SCOPOLAMINE | targetBased | 4 | Completed | 01/10/2021 | https://clinicaltrials.gov/study/NCT04785118 | 1 | LoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Vomiting | SCOPOLAMINE | targetBased | 4 | Completed | 01/10/2021 | https://clinicaltrials.gov/study/NCT04785118 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Vomiting | SCOPOLAMINE | targetBased | 4 | Completed | 01/10/2021 | https://clinicaltrials.gov/study/NCT04785118 | 1 | LoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | PROPANTHELINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=84a920c8-b637-4741-b9b2-fc607e245ce0 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 2 | Completed | 01/07/2003 | https://clinicaltrials.gov/study/NCT01367548 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 2 | Completed | 01/07/2003 | https://clinicaltrials.gov/study/NCT01367548 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 2 | Completed | 21/02/2019 | https://clinicaltrials.gov/study/NCT03852524 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 2 | Completed | 21/02/2019 | https://clinicaltrials.gov/study/NCT03852524 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 3 | Completed | 17/10/2007 | https://clinicaltrials.gov/study/NCT00528970 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 3 | Completed | 17/10/2007 | https://clinicaltrials.gov/study/NCT00528970 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 3 | Completed | 31/10/2006 | https://clinicaltrials.gov/study/NCT00401375 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE | targetBased | 3 | Completed | 31/10/2006 | https://clinicaltrials.gov/study/NCT00401375 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | METHYLNALTREXONE | targetBased | 4 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01012960 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | METHYLNALTREXONE | targetBased | 4 | Completed | 01/11/2009 | https://clinicaltrials.gov/study/NCT01012960 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastroparesis | METHYLNALTREXONE | targetBased | 2 | Terminated | 01/05/2010 | https://clinicaltrials.gov/study/NCT01117376 | 0.2 | LoF | protect | The study was prematurely terminated because of unavailibility of Methylnaltrexone in the region |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastroparesis | METHYLNALTREXONE | targetBased | 2 | Terminated | 01/05/2010 | https://clinicaltrials.gov/study/NCT01117376 | 0.2 | LoF | protect | The study was prematurely terminated because of unavailibility of Methylnaltrexone in the region |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | METHYLNALTREXONE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00387309 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | METHYLNALTREXONE | targetBased | 3 | Completed | 01/12/2006 | https://clinicaltrials.gov/study/NCT00387309 | 0.7 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | ileus | ASIMADOLINE | targetBased | 2 | Terminated | 01/01/2007 | https://clinicaltrials.gov/study/NCT00443040 | 0.2 | GoF | protect | poor enrollment (31 of a planned 114 subjects were randomized and evaluable) |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | irritable bowel syndrome | ASIMADOLINE | targetBased | 3 | Completed | 01/05/2010 | https://clinicaltrials.gov/study/NCT01100684 | 0.7 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | irritable bowel syndrome | ASIMADOLINE | targetBased | 2 | Completed | 01/08/2006 | https://clinicaltrials.gov/study/NCT00454688 | 0.2 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | irritable bowel syndrome | ASIMADOLINE | targetBased | 2 | Completed | 01/01/2005 | https://clinicaltrials.gov/study/NCT00955994 | 0.2 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE HYDROCHLORIDE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE HYDROCHLORIDE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE HYDROCHLORIDE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE HYDROCHLORIDE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | radiation-induced gastrointestinal mucositis | PILOCARPINE HYDROCHLORIDE | targetBased | 3 | Completed | 01/03/1998 | https://clinicaltrials.gov/study/NCT00003139 | 0.7 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) agonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Dysphagia | BUSPIRONE HYDROCHLORIDE | targetBased | 4 | Recruiting | 06/07/2021 | https://clinicaltrials.gov/study/NCT05629325 | 1 | GoF | protect | |
| Primary HTS assay for 5-Hydroxytryptamine (Serotonin) Receptor Subtype 1a (5HT1a) antagonists | HTR1A | HTR1A | 5-hydroxytryptamine receptor 1A | Dysphagia | BUSPIRONE HYDROCHLORIDE | targetBased | 4 | Recruiting | 06/07/2021 | https://clinicaltrials.gov/study/NCT05629325 | 1 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=676078a3-13e5-cc32-e053-2a91aa0ac75f | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | irritable bowel syndrome | DICYCLOMINE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=058b8aa0-0c23-430a-a8fd-0191106fd9e8 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | ileus | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/10/2018 | https://clinicaltrials.gov/study/NCT05669781 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroenteritis | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01165866 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 3 | Terminated | 01/09/2009 | https://clinicaltrials.gov/study/NCT00869310 | 0.7 | LoF | protect | we terminated the study before enrolling 303/560 due to a slow accrual |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | Vomiting | METOCLOPRAMIDE HYDROCHLORIDE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=94e5acdf-f19c-749a-e053-2995a90ae451 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM BROMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM BROMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM BROMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM BROMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | CLIDINIUM BROMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03CA02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRROLATE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | CODEINE PHOSPHATE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | gastrointestinal disease | CODEINE PHOSPHATE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | gastrointestinal disease | CODEINE PHOSPHATE | targetBased | 4 | Completed | 07/09/2017 | https://clinicaltrials.gov/study/NCT03784105 | 1 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ISOPROPAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB09 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ISOPROPAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB09 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ISOPROPAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB09 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ISOPROPAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB09 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | ISOPROPAMIDE | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB09 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB03 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB03 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB03 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB53 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB53 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB53 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB53 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | OXYPHENONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB53 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | HEXOCYCLIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB10 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | HEXOCYCLIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB10 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | HEXOCYCLIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB10 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | HEXOCYCLIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB10 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | HEXOCYCLIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB10 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | GLYCOPYRRONIUM | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB02 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DIPHEMANIL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB15 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DIPHEMANIL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB15 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DIPHEMANIL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB15 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DIPHEMANIL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB15 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | DIPHEMANIL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB15 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | TRIDIHEXETHYL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB08 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | TRIDIHEXETHYL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB08 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | TRIDIHEXETHYL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB08 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | TRIDIHEXETHYL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB08 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | gastrointestinal disease | TRIDIHEXETHYL | targetBased | 4 | https://www.whocc.no/atc_ddd_index/?code=A03AB08 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE BROMIDE | targetBased | 3 | Completed | 17/10/2007 | https://clinicaltrials.gov/study/NCT00528970 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | METHYLNALTREXONE BROMIDE | targetBased | 3 | Completed | 17/10/2007 | https://clinicaltrials.gov/study/NCT00528970 | 0.7 | LoF | protect | |
| Factor XIIa 1536 HTS | F12_modulation | F12 | Coagulation factor XII | flatulence | BISMUTH SUBGALLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fb6ad16b-caac-4a63-a10e-99808a6a83e7 | 1 | GoF | protect | |||
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | ITGA4 | Integrin alpha-4 | pouchitis | VEDOLIZUMAB | targetBased | 3 | Not yet recruiting | 25/06/2024 | https://clinicaltrials.gov/study/NCT06443502 | 0.7 | LoF | protect | |
| HTS for Identification of VLA-4 Allosteric Modulators from MLPCN library | ITGA4 | ITGA4 | Integrin alpha-4 | pouchitis | VEDOLIZUMAB | targetBased | 4 | Completed | 12/10/2016 | https://clinicaltrials.gov/study/NCT02790138 | 1 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/05/2012 | https://clinicaltrials.gov/study/NCT01507233 | 1 | GoF | protect | Slow enrollment. |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/05/2012 | https://clinicaltrials.gov/study/NCT01507233 | 1 | GoF | protect | Slow enrollment. |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/03/2012 | https://clinicaltrials.gov/study/NCT01507220 | 1 | GoF | protect | Slow enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/03/2012 | https://clinicaltrials.gov/study/NCT01507220 | 1 | GoF | protect | Slow enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Completed | 01/12/2011 | https://clinicaltrials.gov/study/NCT01507246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Completed | 01/12/2011 | https://clinicaltrials.gov/study/NCT01507246 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/12/2011 | https://clinicaltrials.gov/study/NCT02058290 | 1 | GoF | protect | Slower than expected enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | intestinal obstruction | MORPHINE SULFATE | targetBased | 4 | Terminated | 01/12/2011 | https://clinicaltrials.gov/study/NCT02058290 | 1 | GoF | protect | Slower than expected enrollment |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALDEMEDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a1256c-a1eb-4abe-ab1e-30e4711afd16 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALDEMEDINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a1256c-a1eb-4abe-ab1e-30e4711afd16 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | NALDEMEDINE | targetBased | 2 | Completed | 19/05/2010 | https://clinicaltrials.gov/study/NCT01122030 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | bowel dysfunction | NALDEMEDINE | targetBased | 2 | Completed | 19/05/2010 | https://clinicaltrials.gov/study/NCT01122030 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | irritable bowel syndrome | ITOPRIDE | targetBased | 2 | Completed | 01/06/2008 | https://clinicaltrials.gov/study/NCT01027260 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastrointestinal disease | ITOPRIDE | targetBased | 3 | https://www.whocc.no/atc_ddd_index/?code=A03FA07 | 0.7 | LoF | protect | |||
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112177 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/09/2004 | https://clinicaltrials.gov/study/NCT00110968 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 2 | Completed | 01/12/2000 | https://clinicaltrials.gov/study/NCT00272103 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Completed | 22/02/2013 | https://clinicaltrials.gov/study/NCT04647955 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/11/2004 | https://clinicaltrials.gov/study/NCT00112203 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 3 | Completed | 01/07/2004 | https://clinicaltrials.gov/study/NCT00112190 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | dyspepsia | ITOPRIDE | targetBased | 4 | Recruiting | 18/11/2021 | https://clinicaltrials.gov/study/NCT04918017 | 1 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | peritonitis | ITOPRIDE | targetBased | 3 | Recruiting | 01/12/2018 | https://clinicaltrials.gov/study/NCT04161768 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | Terminated | 20/08/2020 | https://clinicaltrials.gov/study/NCT04486326 | 1 | LoF | protect | low enrollment |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | Terminated | 20/08/2020 | https://clinicaltrials.gov/study/NCT04486326 | 1 | LoF | protect | low enrollment |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202292s000lbl.pdf | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202292s000lbl.pdf | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 3 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT00547898 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 3 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT00547898 | 0.7 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00002408 | 0.7 | LoF | protect | ||
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00002408 | 0.7 | LoF | protect | ||
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45584fba-8081-4cd1-a21e-3ea8237f62ff | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Diarrhea | CROFELEMER | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45584fba-8081-4cd1-a21e-3ea8237f62ff | 1 | LoF | protect | |||
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | Chronic diarrhea | CROFELEMER | targetBased | 4 | Recruiting | 01/04/2019 | https://clinicaltrials.gov/study/NCT03898856 | 1 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | Chronic diarrhea | CROFELEMER | targetBased | 4 | Recruiting | 01/04/2019 | https://clinicaltrials.gov/study/NCT03898856 | 1 | LoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00002408 | 0.7 | LoF | protect | ||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 3 | Completed | https://clinicaltrials.gov/study/NCT00002408 | 0.7 | LoF | protect | ||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 3 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT00547898 | 0.7 | LoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 3 | Completed | 01/10/2007 | https://clinicaltrials.gov/study/NCT00547898 | 0.7 | LoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202292s000lbl.pdf | 1 | LoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202292s000lbl.pdf | 1 | LoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45584fba-8081-4cd1-a21e-3ea8237f62ff | 1 | LoF | protect | |||
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=45584fba-8081-4cd1-a21e-3ea8237f62ff | 1 | LoF | protect | |||
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | Terminated | 20/08/2020 | https://clinicaltrials.gov/study/NCT04486326 | 1 | LoF | protect | low enrollment |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Diarrhea | CROFELEMER | targetBased | 4 | Terminated | 20/08/2020 | https://clinicaltrials.gov/study/NCT04486326 | 1 | LoF | protect | low enrollment |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | Chronic diarrhea | CROFELEMER | targetBased | 4 | Recruiting | 01/04/2019 | https://clinicaltrials.gov/study/NCT03898856 | 1 | LoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | Chronic diarrhea | CROFELEMER | targetBased | 4 | Recruiting | 01/04/2019 | https://clinicaltrials.gov/study/NCT03898856 | 1 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that inhibit/block calcium-activated chloride channels (TMEM16A) | ANO1_inhibitors | ANO1 | Anoctamin-1 | irritable bowel syndrome | CROFELEMER | targetBased | 2 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00461526 | 0.2 | LoF | protect | |
| Primary cell-based high-throughput screening for identification of compounds that activate/potentiate calcium-activated chloride channels (TMEM16A) | ANO1_activators | ANO1 | Anoctamin-1 | irritable bowel syndrome | CROFELEMER | targetBased | 2 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00461526 | 0.2 | LoF | protect | |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | irritable bowel syndrome | CROFELEMER | targetBased | 2 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00461526 | 0.2 | LoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | irritable bowel syndrome | CROFELEMER | targetBased | 2 | Completed | 01/10/2006 | https://clinicaltrials.gov/study/NCT00461526 | 0.2 | LoF | protect | |
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn6) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | FGB | Fibrinogen beta chain [Cleaved into: Fibrinopeptide B; Fibrinogen beta chain] | gastrointestinal disease | FIBRINOGEN, HUMAN | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01589822 | 0.2 | protect | ||
| Fluorescence polarization-based biochemical primary high throughput screening assay to identify inhibitors that disrupt the binding of a cyclic peptide (Tn7) to the fibrin proteolytic product D-Dimer and fragment E complex [DD(E )] | FGB_inhibitors | FGB | Fibrinogen beta chain [Cleaved into: Fibrinopeptide B; Fibrinogen beta chain] | gastrointestinal disease | FIBRINOGEN, HUMAN | targetBased | 2 | Completed | 01/06/2012 | https://clinicaltrials.gov/study/NCT01589822 | 0.2 | protect | ||
| Counterscreen for Oxytocin Receptor (OXTR) agonists: Fluorescence-based primary cell-based high throughput assay to identify agonists of the vasopressin 1 receptor (V1R) | AVPR1A_agonists | AVPR1A | Vasopressin V1a receptor | esophageal varices | TERLIPRESSIN | targetBased | 4 | Completed | 01/02/2014 | https://clinicaltrials.gov/study/NCT02119884 | 1 | protect | ||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=78e42769-061f-4973-ac3a-dc3fcc4bf641 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=786b9c9a-8b70-41bb-9ea3-0bf6dd7a06df | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=559269eb-4a0f-4b38-8d7d-70d419b96a0e | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | Peptic ulcer | ATROPINE SULFATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=350dcd54-857d-4a65-9fd0-ad54f090fd7b | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | diarrheal disease | ELUXADOLINE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/truberzi | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | diarrheal disease | ELUXADOLINE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/truberzi | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Completed | 29/05/2012 | https://clinicaltrials.gov/study/NCT01553747 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Completed | 29/05/2012 | https://clinicaltrials.gov/study/NCT01553747 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7821bd40-4c84-4984-951b-6436ae20421a | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7821bd40-4c84-4984-951b-6436ae20421a | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Completed | 29/05/2012 | https://clinicaltrials.gov/study/NCT01553591 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Completed | 29/05/2012 | https://clinicaltrials.gov/study/NCT01553591 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 2 | Recruiting | 15/11/2017 | https://clinicaltrials.gov/study/NCT03339128 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 2 | Recruiting | 15/11/2017 | https://clinicaltrials.gov/study/NCT03339128 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 2 | Completed | 28/04/2010 | https://clinicaltrials.gov/study/NCT01130272 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 2 | Completed | 28/04/2010 | https://clinicaltrials.gov/study/NCT01130272 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/truberzi | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | https://www.ema.europa.eu/en/medicines/human/EPAR/truberzi | 1 | GoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | Completed | 23/02/2018 | https://clinicaltrials.gov/study/NCT03441581 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | Completed | 23/02/2018 | https://clinicaltrials.gov/study/NCT03441581 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Enrolling by invitation | 13/08/2021 | https://clinicaltrials.gov/study/NCT04880876 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 3 | Enrolling by invitation | 13/08/2021 | https://clinicaltrials.gov/study/NCT04880876 | 0.7 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | Completed | 25/10/2016 | https://clinicaltrials.gov/study/NCT02959983 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ELUXADOLINE | targetBased | 4 | Completed | 25/10/2016 | https://clinicaltrials.gov/study/NCT02959983 | 1 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALOXEGOL OXALATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c02665d-05d2-4e99-b403-c270de543ebe | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALOXEGOL OXALATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c02665d-05d2-4e99-b403-c270de543ebe | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALOXEGOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c02665d-05d2-4e99-b403-c270de543ebe | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALOXEGOL | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5c02665d-05d2-4e99-b403-c270de543ebe | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | NALOXEGOL | targetBased | 3 | Completed | 14/10/2020 | https://clinicaltrials.gov/study/NCT04433390 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | ileus | NALOXEGOL | targetBased | 3 | Completed | 14/10/2020 | https://clinicaltrials.gov/study/NCT04433390 | 0.7 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALDEMEDINE TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a1256c-a1eb-4abe-ab1e-30e4711afd16 | 1 | LoF | protect | |||
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | constipation disorder | NALDEMEDINE TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b1a1256c-a1eb-4abe-ab1e-30e4711afd16 | 1 | LoF | protect | |||
| Primary Cell-Based Assay to Identify Agonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | S1PR4 | Sphingosine 1-phosphate receptor 4 | eosinophilic esophagitis | ETRASIMOD | targetBased | 2 | Completed | 15/12/2020 | https://clinicaltrials.gov/study/NCT04682639 | 0.2 | protect | ||
| Primary Cell-Based Assay to Identify Antagonists of the Sphingosine 1-Phosphate Receptor 4 (S1P4) | S1PR4 | S1PR4 | Sphingosine 1-phosphate receptor 4 | eosinophilic esophagitis | ETRASIMOD | targetBased | 2 | Completed | 15/12/2020 | https://clinicaltrials.gov/study/NCT04682639 | 0.2 | protect | ||
| Primary HTS and Confirmation Assays for S1P1 Agonists and Agonism Potentiators | S1PR1 | S1PR1 | Sphingosine 1-phosphate receptor 1 | eosinophilic esophagitis | ETRASIMOD | targetBased | 2 | Completed | 15/12/2020 | https://clinicaltrials.gov/study/NCT04682639 | 0.2 | GoF | protect | |
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 1 (CHRM1) | CHRM1_agonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Discovery of Novel Allosteric Modulators of the M1 Muscarinic Receptor: Agonist Primary Screen | CHRM1_allosteric_activators | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human M1 muscarinic receptor (CHRM1). | CHRM1_PAMs | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Discovery of novel allosteric modulators of the M1 muscarinic receptor: Antagonist Primary Screen | CHRM1_allosteric_antagonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human M1 muscarinic receptor (CHRM1) | CHRM1_antgonists | CHRM1 | Muscarinic acetylcholine receptor M1 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 5 (CHRM5) | CHRM5 | CHRM5 | Muscarinic acetylcholine receptor M5 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2189f3f5-39dc-4e85-b7c6-9515ded59686 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=26cdd84d-ffa0-4e52-b359-4acef4bd8093 | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify positive allosteric modulators (PAMs) of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_PAMs | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify antagonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_antgonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| Fluorescence-based cell-based primary high throughput screening assay to identify agonists of the human cholinergic receptor, muscarinic 4 (CHRM4) | CHRM4_agonists | CHRM4 | Muscarinic acetylcholine receptor M4 | peptic ulcer disease | GLYCOPYRRONIUM TOSYLATE | targetBased | 4 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=38f88829-a226-4810-866c-1b62c47e8aee | 1 | LoF | protect | |||
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | pouchitis | LIRAGLUTIDE | targetBased | 2 | Completed | 22/03/2022 | https://clinicaltrials.gov/study/NCT04763564 | 0.2 | GoF | protect | |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | pouchitis | LIRAGLUTIDE | targetBased | 2 | Completed | 22/03/2022 | https://clinicaltrials.gov/study/NCT04763564 | 0.2 | GoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | pouchitis | LIRAGLUTIDE | targetBased | 2 | Completed | 22/03/2022 | https://clinicaltrials.gov/study/NCT04763564 | 0.2 | GoF | protect | |
| qHTS of GLP-1 Receptor Inverse Agonists (Inhibition Mode) | GLP1R | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | LIRAGLUTIDE | targetBased | 2 | Withdrawn | 20/10/2017 | https://clinicaltrials.gov/study/NCT03371862 | 0.2 | GoF | protect | No participants recruited. Not able to recruit due to COVID 19. |
| qHTS of GLP-1 Receptor Agonists | GLP1R agonists | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | LIRAGLUTIDE | targetBased | 2 | Withdrawn | 20/10/2017 | https://clinicaltrials.gov/study/NCT03371862 | 0.2 | GoF | protect | No participants recruited. Not able to recruit due to COVID 19. |
| qHTS of GLP-1 Receptor Inverse Agonists: (PAM Mode) (Taking the negative queue for PAMs) | GLP1R PAMs | GLP1R | Glucagon-like peptide 1 receptor , Glucagon-like peptide-1 receptor | short bowel syndrome | LIRAGLUTIDE | targetBased | 2 | Withdrawn | 20/10/2017 | https://clinicaltrials.gov/study/NCT03371862 | 0.2 | GoF | protect | No participants recruited. Not able to recruit due to COVID 19. |
| Identification of Small Molecule Correctors of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Delta508 Mutation Function in Human Bronchial Epithelial Cells. Measured in Cell-Based System Using Plate Reader - 7017-01_Other_SinglePoint_HTS_Activity | cftrCorrectors | CFTR | Cystic fibrosis transmembrane conductance regulator | gastrointestinal disease | IOWH-032 | targetBased | 2 | Completed | 04/11/2019 | https://clinicaltrials.gov/study/NCT04150250 | 0.2 | LoF | protect | |
| Fluorescence Polarization with CAL-PDZ Measured in Biochemical System Using Plate Reader - 2109-02_Inhibitor_SinglePoint_HTS_Activity | cftrTrafficModulators | CFTR | Cystic fibrosis transmembrane conductance regulator | gastrointestinal disease | IOWH-032 | targetBased | 2 | Completed | 04/11/2019 | https://clinicaltrials.gov/study/NCT04150250 | 0.2 | LoF | protect | |
| uHTS identification of small molecule antagonists of the kappa opioid receptor via a luminescent beta-arrestin assay | OPRK1 | OPRK1 | Kappa-type opioid receptor | irritable bowel syndrome | ORP-101 | targetBased | 2 | Completed | 22/11/2019 | https://clinicaltrials.gov/study/NCT04129619 | 0.2 | LoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ORP-101 | targetBased | 2 | Completed | 22/11/2019 | https://clinicaltrials.gov/study/NCT04129619 | 0.2 | GoF | protect | |
| Luminescence-based cell-based primary high throughput screening assay to identify inverse agonists of heterodimerization of the mu 1 (OPRM1) and delta 1 (OPRD1) opioid receptors | OPRM1 | OPRM1 | Mu-type opioid receptor | irritable bowel syndrome | ORP-101 | targetBased | 2 | Completed | 22/11/2019 | https://clinicaltrials.gov/study/NCT04129619 | 0.2 | GoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Agonists of the Human D2 Dopamine Receptor: Primary Screen for Agonists | D2_agonists | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| HTS Assay for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Primary Screen for Potentiators | D2_PAMs | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| Beta-Arrestin HTS for Positive Allosteric Modulators of the Human D2 Dopamine Receptor: Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| HTS Assay for Allosteric Antagonists of the Human D2 Dopamine Receptor: Primary Screen for Antagonists | D2_antagonists | DRD2 | Dopaminereceptor2 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Antagonist: qHTS | D3_antagonists | DRD3 | Dopaminereceptor3 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Agonist: qHTS | D3_agonists | DRD3 | Dopaminereceptor3 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect | |
| qHTS of D3 Dopamine Receptor Potentiators: qHTS | D3_PAMs | DRD3 | Dopaminereceptor3 | gastroparesis | TRAZPIROBEN | targetBased | 2 | Completed | 14/10/2018 | https://clinicaltrials.gov/study/NCT03544229 | 0.2 | LoF | protect |