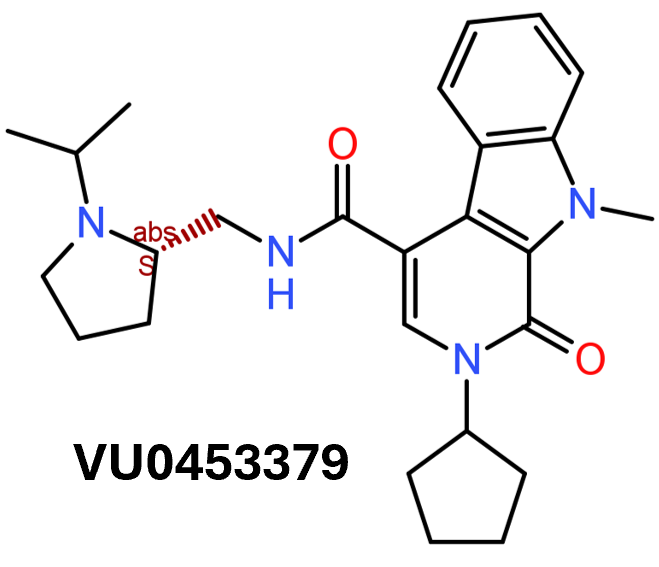
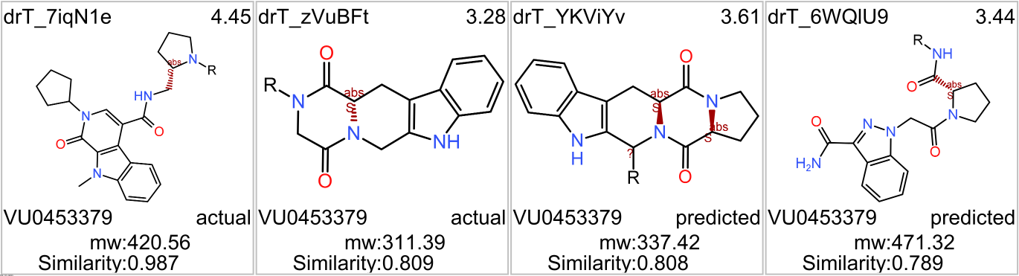
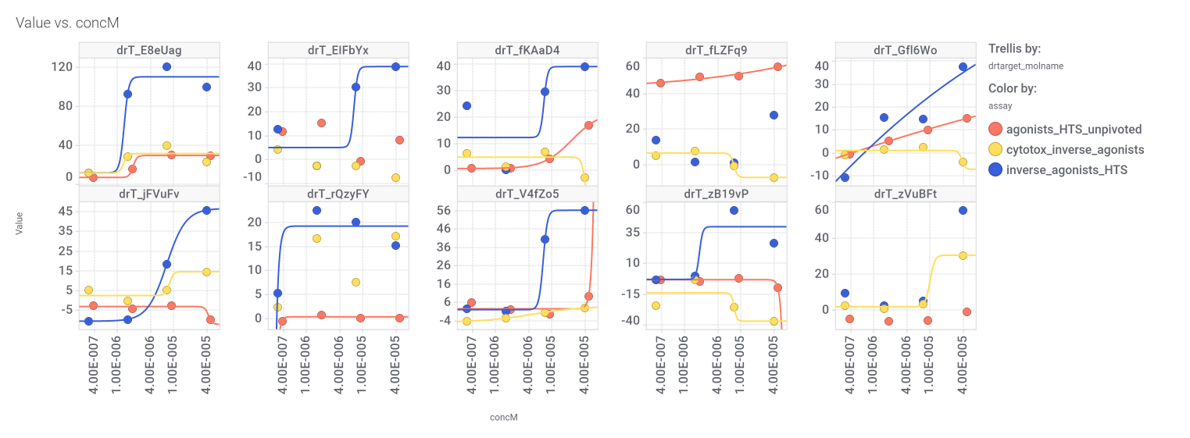
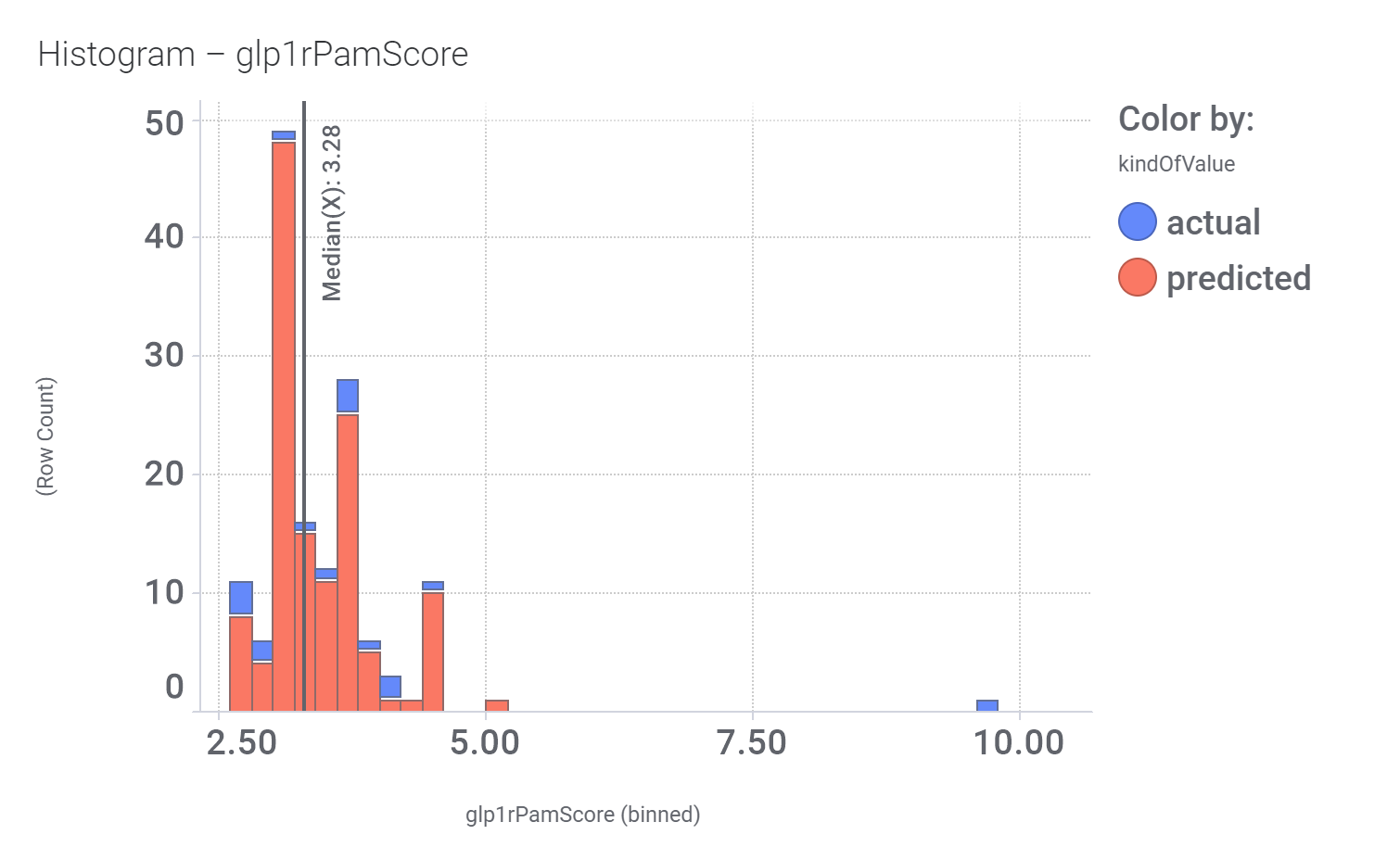
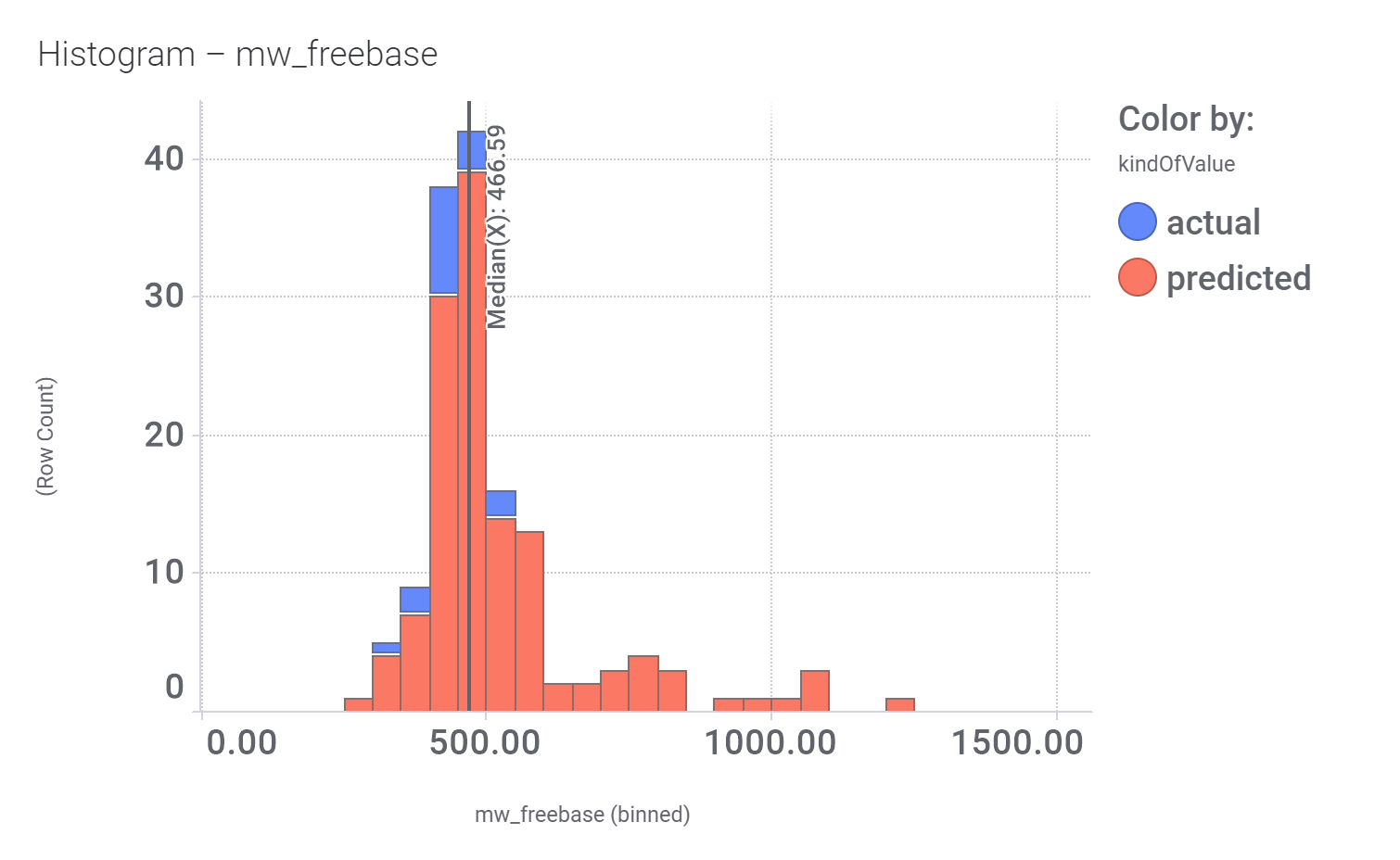
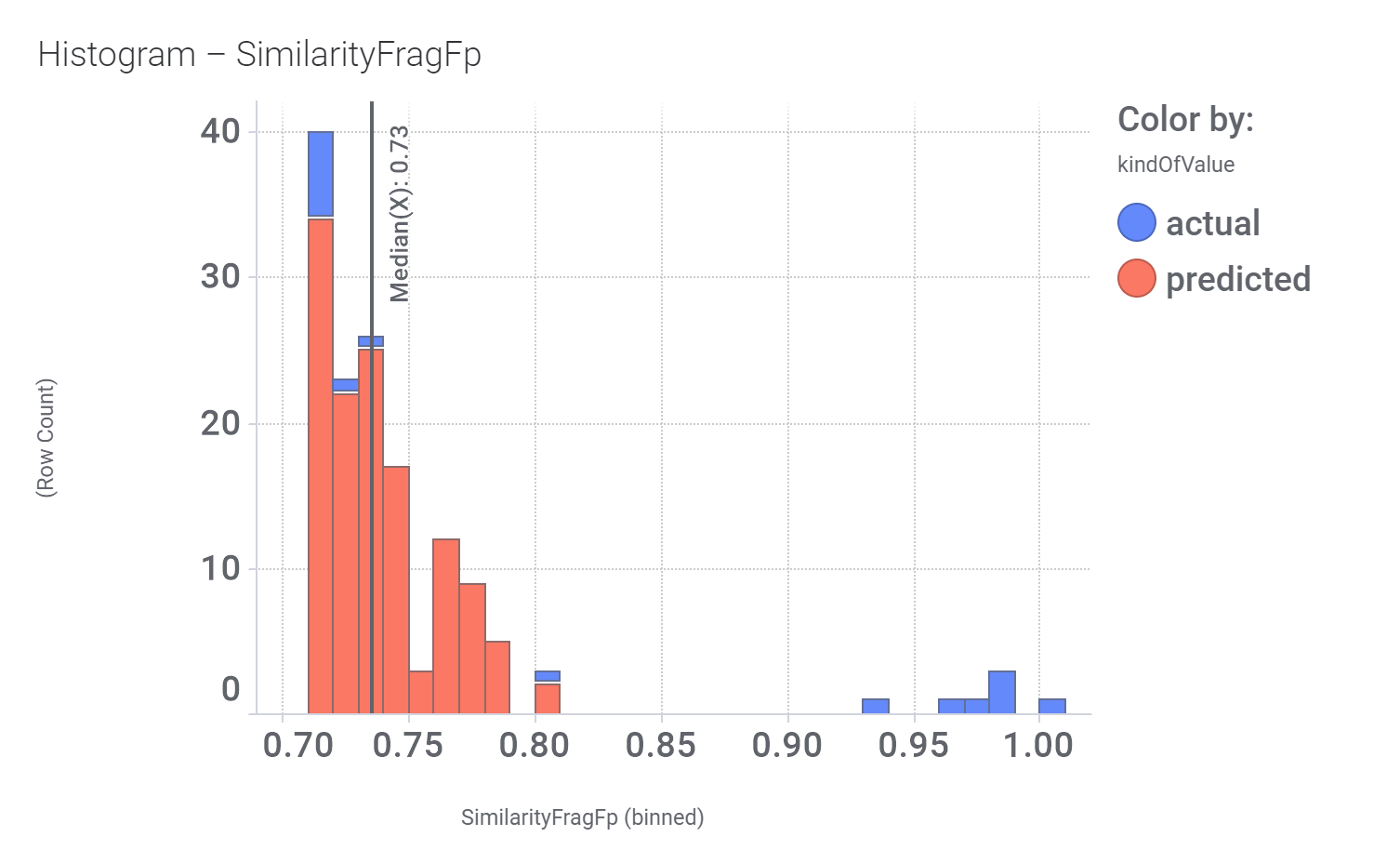
In 2014, an HTS carried out in Vanderbilt University School of Medicine, followed by SAR and optimization of chemical seriesled to the identification of VU0453379, a pyridoindolamide with in vitro activity and in vivo efficacy. DrTarget has predicted GLP1R PAM activity for 145 compounds with > 70% similarity with VU0453379.
See exemplars.
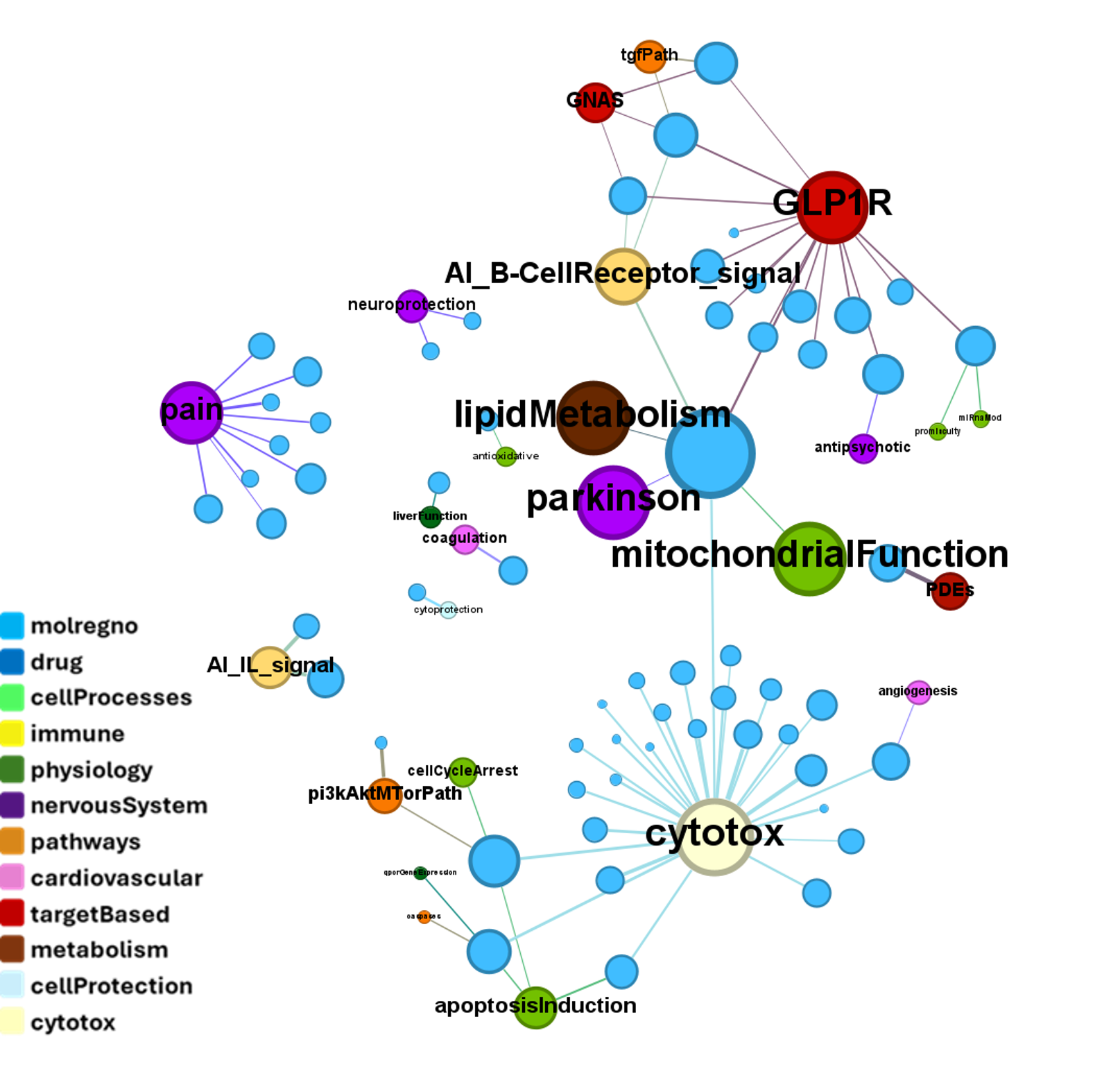
Interactions of such compounds with relevant phenotypes recorded at ChEMBL can be visualized with a network graph
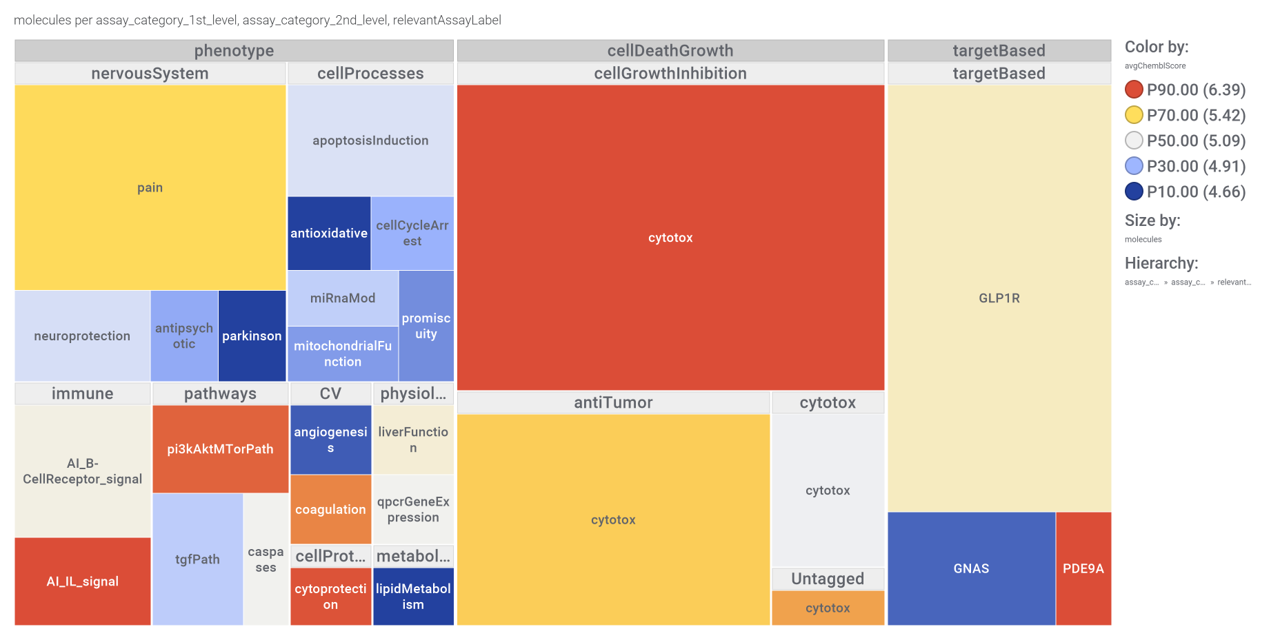
Or a treeMap
Some of these compounds show activity in GLP1R PChem screens
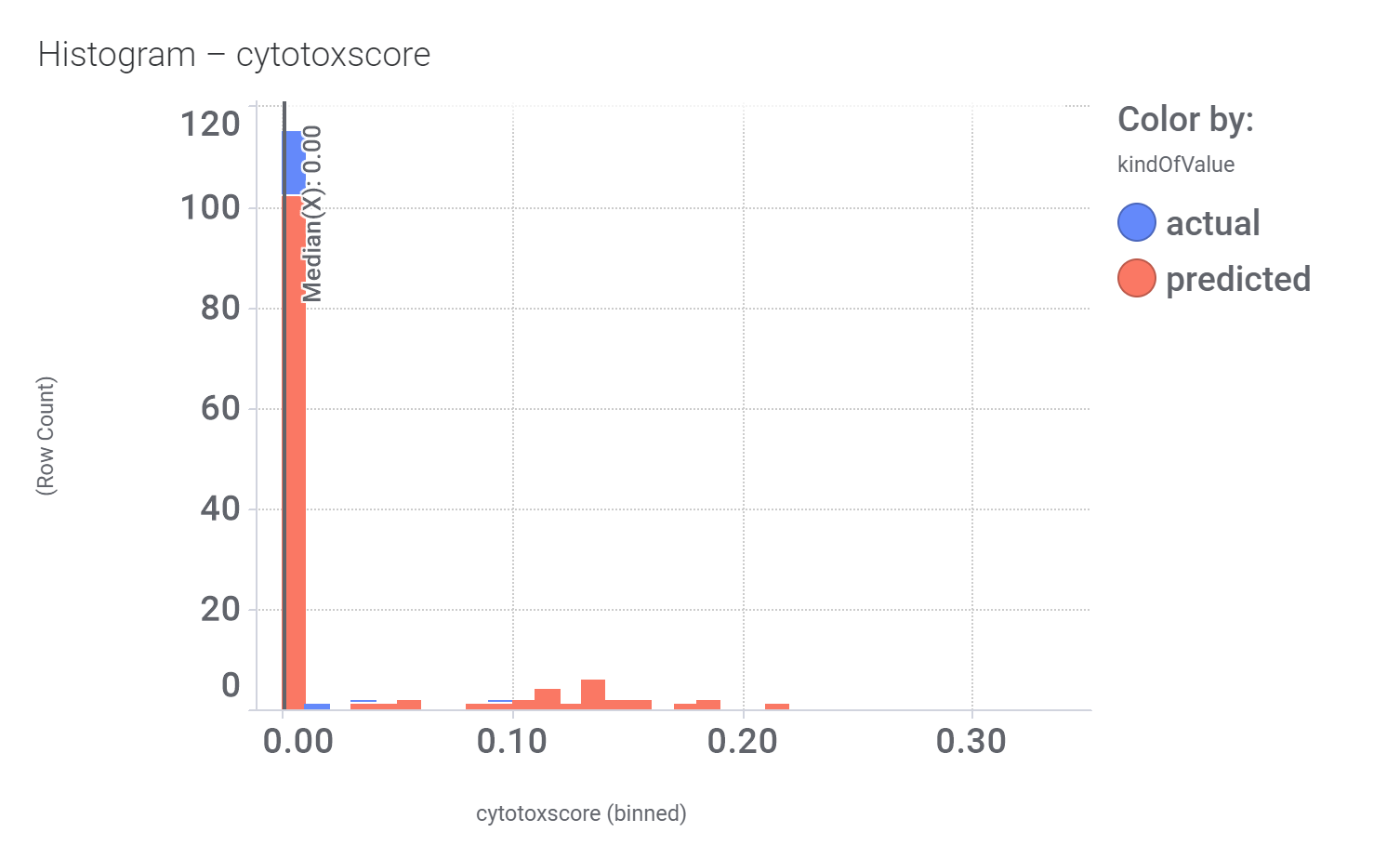
Bio-physicochemical and developability properties
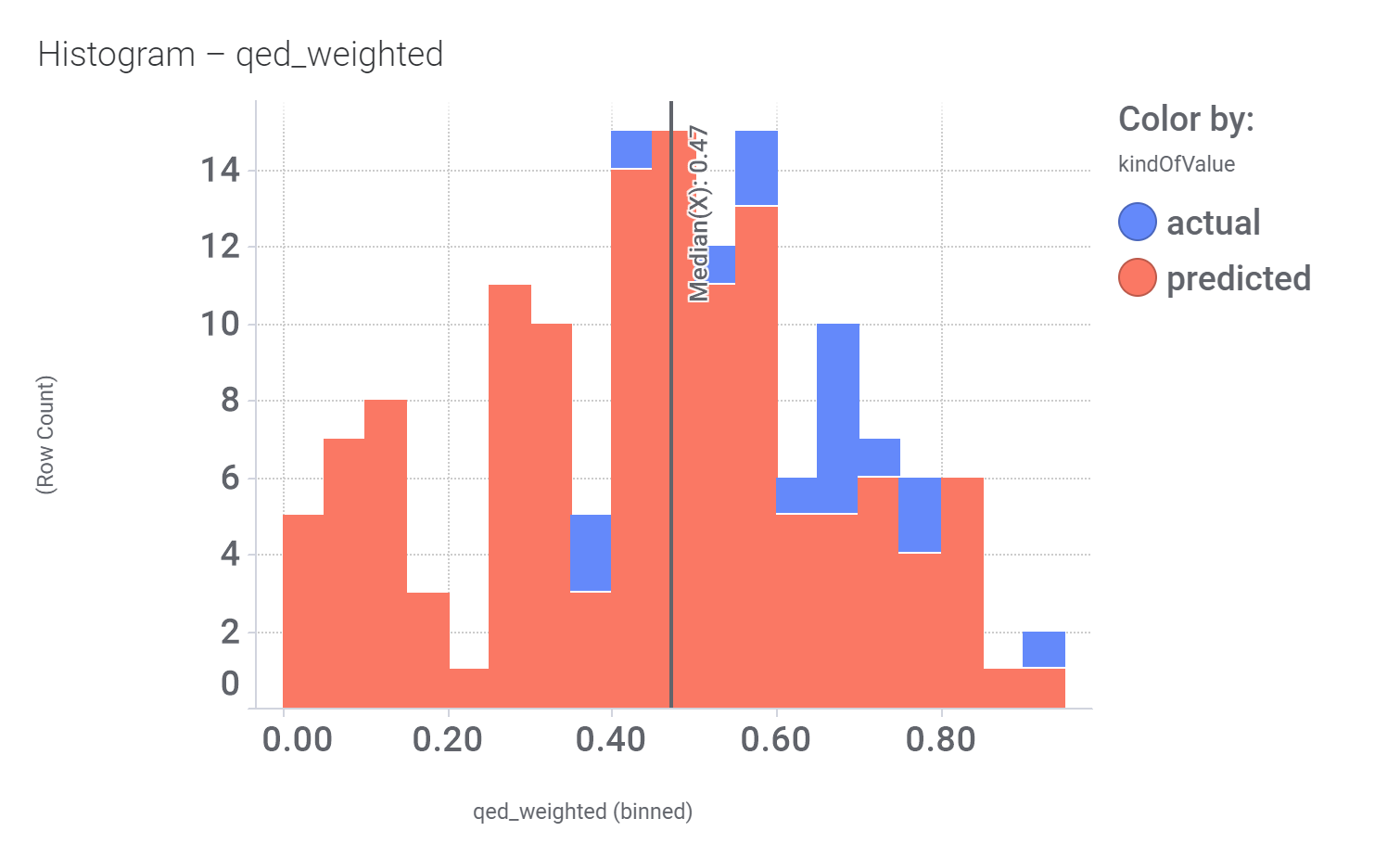
Druglikeness estimated by QedWeighted
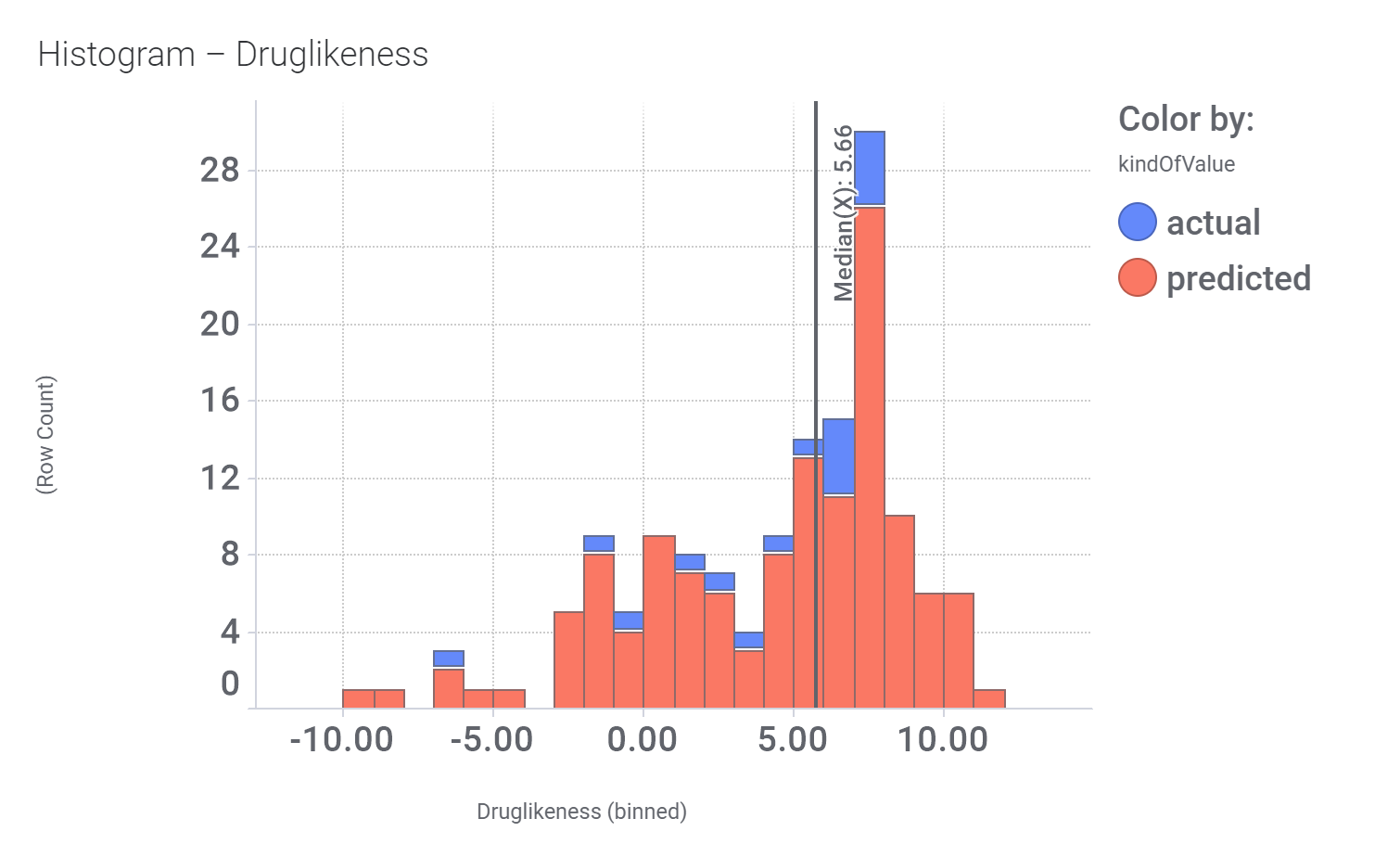
Druglikeness estimated by DataWarrior
literature references
Br J Pharmacol. 2022 Feb; 179(4): 511–525. doi: 10.1111/bph.15446
Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: Alternative approaches for treatment of Type 2 diabetes
Faisal Malik 1 and Zhijun Li 1
Abstract
Glucagon‐like peptide‐1 (GLP‐1) receptors belong to the pharmaceutically important Class B family of GPCRs and are involved in many biologically significant signalling pathways. Its incretin peptide ligand GLP‐1 analogues are effective treatments for Type 2 diabetes. Although developing non‐peptide low MW drugs targeting GLP‐1 receptors remains elusive, considerable progress has been made in discovering non‐peptide agonists and positive allosteric modulators (PAMs) of GLP‐1 receptors with demonstrated efficacy. Many of these compounds induce biased signalling in GLP‐1 receptor‐mediated functional pathways. High‐quality structures of GLP‐1 receptors in both inactive and active states have been reported, revealing detailed molecular interactions between GLP‐1 receptors and non‐peptide agonists or PAMs. These progresses raise the exciting possibility of developing non‐peptide drugs of GLP‐1 receptors as alternative treatments for Type 2 diabetes. The insight into the interactions between the receptor and the non‐peptide ligand is also useful for developing non‐peptide ligands targeting other Class B GPCRs.
3.1.5. Compound VU0453379
Morris, Days, et al. (2014) developed an innovative HT screening system based on a primary Ca2+ screen, a secondary cAMP screen and follow‐up testing to identify non‐peptide agonists and PAMs of human GLP‐1 receptors. Out of 175,000 compounds initially screened, 98 compounds showed a variety of activities at GLP‐1 receptors. Among them, VU00056556 and VU0109197, which share a common hexahydroquinolone carboxylate core, were PAMS for GLP‐1 receptors. Both compounds induced GLP‐1‐dependent cAMP accumulation and Ca2+ mobilization within the human 9‐3‐H cells expressing GLP‐1 receptors at a higher level than GLP‐1 alone. In addition, several other compounds can modulate GLP‐1 receptor activities.
Following the HT screening, the group selected one lead compound, separated its racemic isomers and further optimized the structure of its (S)‐enantiomer. This was followed by SAR studies using an analogue library. All these efforts led to the discovery of a novel PAM, compound 12 (VU0453379) (Figure 3, [12]) (Morris, Nance, et al., 2014). VU0453379 exhibits no probe dependence by potentiating endogenous GLP‐1 as well as synthetic peptide agonists, exendin 4 and liragulitide. VU0453379 shows biased signalling with weak effects on β‐arrestin recruitment and is highly selective towards GLP‐1 receptors. In primary mouse pancreatic islets, VU0453379 augmented glucose‐dependent insulin secretion by low‐dose exendin‐4. Using a haloperidol‐induced catalepsy model, VU0453379 significantly reversed catalepsy by potentiating endogenous GLP‐1 receptors. With favourable physiochemical properties, VU0453379 also demonstrates favourable metabolic and pharmokinetic profiles. A rather important observation is that VU0453379 crossed the blood‐brain barrier and was the first CNS‐penetrant GLP‐1 receptor PAM ever reported.
J Med Chem. 2014 Dec 11; 57(23): 10192–10197.
Published online 2014 Nov 25. doi: 10.1021/jm501375c. PMCID: PMC4266362. PMID: 25423411
Discovery of (S)-2-Cyclopentyl-N-((1-isopropylpyrrolidin2-yl)-9-methyl-1-oxo-2,9-dihydro-1H-pyrrido[3,4-b]indole-4-carboxamide (VU0453379): A Novel, CNS Penetrant Glucagon-Like Peptide 1 Receptor (GLP-1R) Positive Allosteric Modulator (PAM)
Lindsey C. Morris,†∇ Kellie D. Nance,§#∇ Patrick R. Gentry,‡§ Emily L. Days,∥ C. David Weaver,‡∥ Colleen M. Niswender,‡§ Analisa D. Thompson,‡§ Carrie K. Jones,‡§ Chuck W. Locuson,§ Ryan D. Morrison,§ J. Scott Daniels,‡§ Kevin D. Niswender,*⊥† and Craig W. Lindsley*‡§#∥
Abstract
A duplexed, functional multiaddition high throughput screen and subsequent iterative parallel synthesis effort identified the first highly selective and CNS penetrant glucagon-like peptide-1R (GLP-1R) positive allosteric modulator (PAM). PAM (S)-9b potentiated low-dose exenatide to augment insulin secretion in primary mouse pancreatic islets, and (S)-9b alone was effective in potentiating endogenous GLP-1R to reverse haloperidol-induced catalepsy.